Thermal stability of levopimaric acid and its oxidation products
- PMID: 37730608
- PMCID: PMC10512607
- DOI: 10.1186/s13065-023-01031-z
Thermal stability of levopimaric acid and its oxidation products
Abstract
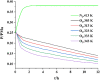
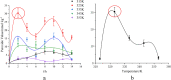
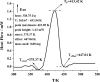
Biofuels are renewable alternatives to fossil fuels. Levopimaric acid‒base biofuels have attracted increasing attention. However, their stability remains a critical issue in practice. Thus, there is a strong impetus to evaluate the thermal stability of levopimaric acid. Through thermogravimetry (TG) and a custom-designed mini closed pressure vessel test (MCPVT) operating under isothermal and stepped temperature conditions, we investigated thermal oxidation characteristics of levopimaric acid under oxygen atmosphere. Thin-layer chromatography (TLC) and iodimetry were used to measure the hydrogen peroxides generated by levopimaric acid oxidation. A high pressure differential scanning calorimeter (HPDSC) was used to assess hydroperoxide thermal decomposition characteristics. Gas chromatography-mass spectrometry (GC-MS) was used to characterize the oxidation products. The thermal decomposition kinetics of levopimaric acid were thus elucidated, and a high peroxide value was detected in the levopimaric acid. The decomposition heat (QDSC) and exothermic onset temperature (Tonset) of hydroperoxides were 338.75 J g-1 and 375.37 K, respectively. Finally, levopimaric acid underwent a second-stage oxidation process at its melt point (423.15 K), resulting in complex oxidation products. Thermal oxidation of levopimaric acid could yield potential thermal hazards, indicating that antioxidants must be added during levopimaric acid application to protect against such hazardous effects.
Keywords: Oxidation characteristics; Peroxide value; Reaction progress; Thermal decomposition; Thermal oxidation.
© 2023. Springer Nature Switzerland AG.
Conflict of interest statement
The authors declare no competing interests.
Figures






Similar articles
-
Oxidation characteristic and thermal runaway of isoprene.BMC Chem. 2023 Sep 2;17(1):110. doi: 10.1186/s13065-023-01016-y. BMC Chem. 2023. PMID: 37660031 Free PMC article.
-
Characteristics and hazards of the cinnamaldehyde oxidation process.RSC Adv. 2020 May 20;10(32):19124-19133. doi: 10.1039/c9ra10820c. eCollection 2020 May 14. RSC Adv. 2020. PMID: 35518288 Free PMC article.
-
Thermal decomposition characteristics of BHT and its peroxide (BHTOOH).BMC Chem. 2024 Apr 29;18(1):87. doi: 10.1186/s13065-024-01190-7. BMC Chem. 2024. PMID: 38685077 Free PMC article.
-
Thermal stability and pathways for the oxidation of four 3-phenyl-2-propene compounds.RSC Adv. 2021 Oct 5;11(52):32654-32670. doi: 10.1039/d1ra04836h. eCollection 2021 Oct 4. RSC Adv. 2021. PMID: 35493582 Free PMC article.
-
The Minderoo-Monaco Commission on Plastics and Human Health.Ann Glob Health. 2023 Mar 21;89(1):23. doi: 10.5334/aogh.4056. eCollection 2023. Ann Glob Health. 2023. PMID: 36969097 Free PMC article. Review.
References
-
- Li Z, Yang X, Liu H, Yang X, Shan Y, Xu X, et al. Dual-functional antimicrobial coating based on a quaternary ammonium salt from rosin acid with in vitro and in vivo antimicrobial and antifouling properties. Chem Eng J. 2019;374:564–75. doi: 10.1016/j.cej.2019.05.208. - DOI
-
- Mei L, Yan Y, Li Z, Ran J, Shen L, Wu R, et al. Identification of the diterpenoid biosynthesis genes and their expression status in relation to oleoresin yield of masson pine. Ind Crop Prod. 2021;170:113827. doi: 10.1016/j.indcrop.2021.113827. - DOI
-
- Mantzaridis C, Brocas AL, Llevot A, Cendejas G, Auvergne R, Caillol S, et al. Rosin acid oligomers as precursors of dgeba-free epoxy resins. Green Chem. 2013;15(11):3091–8. doi: 10.1039/c3gc41004h. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
