Dual inhibition of glycolysis and glutaminolysis for synergistic therapy of rheumatoid arthritis
- PMID: 37730663
- PMCID: PMC10510293
- DOI: 10.1186/s13075-023-03161-0
Dual inhibition of glycolysis and glutaminolysis for synergistic therapy of rheumatoid arthritis
Abstract
Background: Synovial fibroblasts in rheumatoid arthritis (RAFLS) exhibit a pathological aberration of glycolysis and glutaminolysis. Henceforth, we aimed to investigate if dual inhibition of these pathways by phytobiological compound c28MS has the potential of synergistic therapy for arthritis by targeting both glucose and glutamine metabolism.
Methods: The presence of HK2 and GLS across various cell types and associated gene expression in human synovial cells and a murine model of arthritis was evaluated by scRNA-seq. The metabolic profiling of RAFLS cells was done using H1-nuclear magnetic resonance spectroscopy under glycolytic and glutaminolytic inhibitory conditions by incubating with 3-bromopyruvate, CB839, or dual inhibitor c28MS. FLS functional analysis was conducted under similar conditions. ELISA was employed for the quantification of IL-6, CCL2, and MMP3. K/BxN sera was administered to mice to induce arthritis for in vivo arthritis experiments.
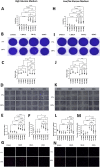
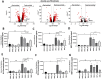
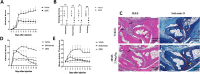
Results: scRNA-seq analysis revealed that many fibroblasts expressed Hk2 along with Gls with several genes including Ptgs2, Hif1a, Timp1, Cxcl5, and Plod2 only associated with double-positive fibroblasts, suggesting that dual inhibition can be an attractive target for fibroblasts. Metabolomic and functional analysis revealed that c28MS decreased the aggressive behavior of RAFLS by targeting both upregulated glycolysis and glutaminolysis. c28MS administered in vivo significantly decreased the severity of arthritis in the K/BxN model.
Conclusion: Our findings imply that dual inhibition of glycolysis and glutaminolysis could be an effective approach for the treatment of RA. It also suggests that targeting more than one metabolic pathway can be a novel treatment approach in non-cancer diseases.
Keywords: Fibroblast-like synoviocytes; Glucose metabolism; Glutaminase; Glutamine metabolism hexokinase; Rheumatoid arthritis.
© 2023. BioMed Central Ltd., part of Springer Nature.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Macfarlane FR, Chaplain MA, Eftimie R. Modelling rheumatoid arthritis: a hybrid modelling framework to describe pannus formation in a small joint. ImmunoInformatics. 2022;6:100014.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

