VE-cadherin in arachnoid and pia mater cells serves as a suitable landmark for in vivo imaging of CNS immune surveillance and inflammation
- PMID: 37730744
- PMCID: PMC10511632
- DOI: 10.1038/s41467-023-41580-4
VE-cadherin in arachnoid and pia mater cells serves as a suitable landmark for in vivo imaging of CNS immune surveillance and inflammation
Abstract
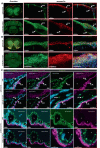
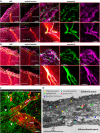
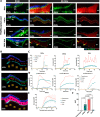
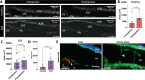
Meninges cover the surface of the brain and spinal cord and contribute to protection and immune surveillance of the central nervous system (CNS). How the meningeal layers establish CNS compartments with different accessibility to immune cells and immune mediators is, however, not well understood. Here, using 2-photon imaging in female transgenic reporter mice, we describe VE-cadherin at intercellular junctions of arachnoid and pia mater cells that form the leptomeninges and border the subarachnoid space (SAS) filled with cerebrospinal fluid (CSF). VE-cadherin expression also marked a layer of Prox1+ cells located within the arachnoid beneath and separate from E-cadherin+ arachnoid barrier cells. In vivo imaging of the spinal cord and brain in female VE-cadherin-GFP reporter mice allowed for direct observation of accessibility of CSF derived tracers and T cells into the SAS bordered by the arachnoid and pia mater during health and neuroinflammation, and detection of volume changes of the SAS during CNS pathology. Together, the findings identified VE-cadherin as an informative landmark for in vivo imaging of the leptomeninges that can be used to visualize the borders of the SAS and thus potential barrier properties of the leptomeninges in controlling access of immune mediators and immune cells into the CNS during health and neuroinflammation.
© 2023. Springer Nature Limited.
Conflict of interest statement
The authors declare no competing interests.
Figures





References
-
- Haines, D. E. & Mihailoff., G. A. Fundamental neuroscience for basic and clinical application. In:Science Direct; Elsevier Publishing Company: Amsterdam, The Netherlands, 107–121 (2018).
-
- Balin BJ, Broadwell RD, Salcman M, el-Kalliny M. Avenues for entry of peripherally administered protein to the central nervous system in mouse, rat, and squirrel monkey. J. Comp. Neurol. 1986;251:260–280. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

