Assessment of safety and intranasal neutralizing antibodies of HPMC-based human anti-SARS-CoV-2 IgG1 nasal spray in healthy volunteers
- PMID: 37730833
- PMCID: PMC10511465
- DOI: 10.1038/s41598-023-42539-7
Assessment of safety and intranasal neutralizing antibodies of HPMC-based human anti-SARS-CoV-2 IgG1 nasal spray in healthy volunteers
Abstract
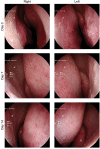
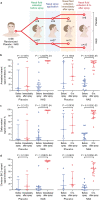
An HPMC-based nasal spray solution containing human IgG1 antibodies against SARS-CoV-2 (nasal antibody spray or NAS) was developed to strengthen COVID-19 management. NAS exhibited potent broadly neutralizing activities against SARS-CoV-2 with PVNT50 values ranging from 0.0035 to 3.1997 μg/ml for the following variants of concern (ranked from lowest to highest): Alpha, Beta, Gamma, ancestral, Delta, Omicron BA.1, BA.2, BA.4/5, and BA.2.75. Biocompatibility assessment showed no potential biological risks. Intranasal NAS administration in rats showed no circulatory presence of human IgG1 anti-SARS-CoV-2 antibodies within 120 h. A double-blind, randomized, placebo-controlled trial (NCT05358873) was conducted on 36 healthy volunteers who received either NAS or a normal saline nasal spray. Safety of the thrice-daily intranasal administration for 7 days was assessed using nasal sinuscopy, adverse event recording, and self-reporting questionnaires. NAS was well tolerated, with no significant adverse effects during the 14 days of the study. The SARS-CoV-2 neutralizing antibodies were detected based on the signal inhibition percent (SIP) in nasal fluids pre- and post-administration using a SARS-CoV-2 surrogate virus neutralization test. SIP values in nasal fluids collected immediately or 6 h after NAS application were significantly increased from baseline for all three variants tested, including ancestral, Delta, and Omicron BA.2. In conclusion, NAS was safe for intranasal use in humans to increase neutralizing antibodies in nasal fluids that lasted at least 6 h.
© 2023. Springer Nature Limited.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

