Cingulate dynamics track depression recovery with deep brain stimulation
- PMID: 37730990
- PMCID: PMC10550829
- DOI: 10.1038/s41586-023-06541-3
Cingulate dynamics track depression recovery with deep brain stimulation
Abstract
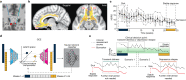
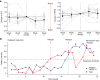
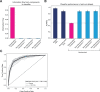
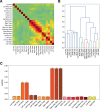
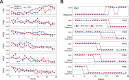
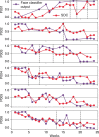
Deep brain stimulation (DBS) of the subcallosal cingulate (SCC) can provide long-term symptom relief for treatment-resistant depression (TRD)1. However, achieving stable recovery is unpredictable2, typically requiring trial-and-error stimulation adjustments due to individual recovery trajectories and subjective symptom reporting3. We currently lack objective brain-based biomarkers to guide clinical decisions by distinguishing natural transient mood fluctuations from situations requiring intervention. To address this gap, we used a new device enabling electrophysiology recording to deliver SCC DBS to ten TRD participants (ClinicalTrials.gov identifier NCT01984710). At the study endpoint of 24 weeks, 90% of participants demonstrated robust clinical response, and 70% achieved remission. Using SCC local field potentials available from six participants, we deployed an explainable artificial intelligence approach to identify SCC local field potential changes indicating the patient's current clinical state. This biomarker is distinct from transient stimulation effects, sensitive to therapeutic adjustments and accurate at capturing individual recovery states. Variable recovery trajectories are predicted by the degree of preoperative damage to the structural integrity and functional connectivity within the targeted white matter treatment network, and are matched by objective facial expression changes detected using data-driven video analysis. Our results demonstrate the utility of objective biomarkers in the management of personalized SCC DBS and provide new insight into the relationship between multifaceted (functional, anatomical and behavioural) features of TRD pathology, motivating further research into causes of variability in depression treatment.
© 2023. The Author(s).
Conflict of interest statement
K.S.C. is a consultant to Abbott Laboratories. H.S.M. received consulting and IP licensing fees from Abbott Laboratories. P.R.-P. has received consulting fees Janssen Pharmaceuticals, Abbott Laboratories and LivaNova, Inc. B.H.K. is a consultant for Abbott Neuromodulation, Medtronic and ClearPoint Neuro. R.E.G. serves as a consultant to Medtronic, which manufactures products related to the research described in this paper. R.E.G. receives compensation for these services. The terms of this arrangement have been reviewed and approved by Emory University and the Icahn School of Medicine at Mount Sinai in accordance with their respective conflict of interest policies. S.A., C.J.R., H.S.M., V.T., P.R.-P., A.C., R.B., A.C.W., K.S.C. and A.V. are listed as inventors on provisional patent filings related to the findings in this manuscript.
Figures






References
-
- Crowell, A. L. et al. Long-term outcomes of subcallosal cingulate deep brain stimulation for treatment-resistant depression. Am. J. Psychiatry10.1176/appi.ajp.2019.18121427 (2019). - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

