Transgenic ferret models define pulmonary ionocyte diversity and function
- PMID: 37730992
- PMCID: PMC10533402
- DOI: 10.1038/s41586-023-06549-9
Transgenic ferret models define pulmonary ionocyte diversity and function
Abstract
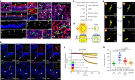
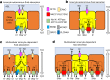
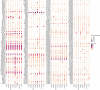
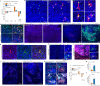
Speciation leads to adaptive changes in organ cellular physiology and creates challenges for studying rare cell-type functions that diverge between humans and mice. Rare cystic fibrosis transmembrane conductance regulator (CFTR)-rich pulmonary ionocytes exist throughout the cartilaginous airways of humans1,2, but limited presence and divergent biology in the proximal trachea of mice has prevented the use of traditional transgenic models to elucidate ionocyte functions in the airway. Here we describe the creation and use of conditional genetic ferret models to dissect pulmonary ionocyte biology and function by enabling ionocyte lineage tracing (FOXI1-CreERT2::ROSA-TG), ionocyte ablation (FOXI1-KO) and ionocyte-specific deletion of CFTR (FOXI1-CreERT2::CFTRL/L). By comparing these models with cystic fibrosis ferrets3,4, we demonstrate that ionocytes control airway surface liquid absorption, secretion, pH and mucus viscosity-leading to reduced airway surface liquid volume and impaired mucociliary clearance in cystic fibrosis, FOXI1-KO and FOXI1-CreERT2::CFTRL/L ferrets. These processes are regulated by CFTR-dependent ionocyte transport of Cl- and HCO3-. Single-cell transcriptomics and in vivo lineage tracing revealed three subtypes of pulmonary ionocytes and a FOXI1-lineage common rare cell progenitor for ionocytes, tuft cells and neuroendocrine cells during airway development. Thus, rare pulmonary ionocytes perform critical CFTR-dependent functions in the proximal airway that are hallmark features of cystic fibrosis airway disease. These studies provide a road map for using conditional genetics in the first non-rodent mammal to address gene function, cell biology and disease processes that have greater evolutionary conservation between humans and ferrets.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures













References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

