Synthesis of portimines reveals the basis of their anti-cancer activity
- PMID: 37730997
- PMCID: PMC10699793
- DOI: 10.1038/s41586-023-06535-1
Synthesis of portimines reveals the basis of their anti-cancer activity
Erratum in
-
Author Correction: Synthesis of portimines reveals the basis of their anti-cancer activity.Nature. 2023 Oct;622(7984):E3. doi: 10.1038/s41586-023-06699-w. Nature. 2023. PMID: 37798445 No abstract available.
Abstract
Marine-derived cyclic imine toxins, portimine A and portimine B, have attracted attention because of their chemical structure and notable anti-cancer therapeutic potential1-4. However, access to large quantities of these toxins is currently not feasible, and the molecular mechanism underlying their potent activity remains unknown until now. To address this, a scalable and concise synthesis of portimines is presented, which benefits from the logic used in the two-phase terpenoid synthesis5,6 along with other tactics such as exploiting ring-chain tautomerization and skeletal reorganization to minimize protecting group chemistry through self-protection. Notably, this total synthesis enabled a structural reassignment of portimine B and an in-depth functional evaluation of portimine A, revealing that it induces apoptosis selectively in human cancer cell lines with high potency and is efficacious in vivo in tumour-clearance models. Finally, practical access to the portimines and their analogues simplified the development of photoaffinity analogues, which were used in chemical proteomic experiments to identify a primary target of portimine A as the 60S ribosomal export protein NMD3.
© 2023. The Author(s), under exclusive licence to Springer Nature Limited.
Figures
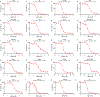
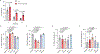
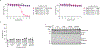



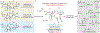



Comment in
-
Synthesizing portimines.Nat Rev Drug Discov. 2023 Nov;22(11):873. doi: 10.1038/d41573-023-00161-2. Nat Rev Drug Discov. 2023. PMID: 37798467 No abstract available.
Similar articles
-
Antifouling activity of portimine, select semisynthetic analogues, and other microalga-derived spirocyclic imines.Biofouling. 2018 Sep;34(8):950-961. doi: 10.1080/08927014.2018.1514461. Epub 2018 Dec 12. Biofouling. 2018. PMID: 30539667
-
Cephalostatin analogues--synthesis and biological activity.Fortschr Chem Org Naturst. 2004;87:1-80. doi: 10.1007/978-3-7091-0581-8_1. Fortschr Chem Org Naturst. 2004. PMID: 15079895 Review.
-
The marine cytotoxin portimine is a potent and selective inducer of apoptosis.Apoptosis. 2016 Dec;21(12):1447-1452. doi: 10.1007/s10495-016-1302-x. Apoptosis. 2016. PMID: 27738771
-
The Spirocyclic Imine from a Marine Benthic Dinoflagellate, Portimine, Is a Potent Anti-Human Immunodeficiency Virus Type 1 Therapeutic Lead Compound.Mar Drugs. 2019 Aug 24;17(9):495. doi: 10.3390/md17090495. Mar Drugs. 2019. PMID: 31450557 Free PMC article.
-
Synthesis and biology of cyclic imine toxins, an emerging class of potent, globally distributed marine toxins.Nat Prod Rep. 2015 Mar;32(3):411-35. doi: 10.1039/c4np00089g. Nat Prod Rep. 2015. PMID: 25338021 Free PMC article. Review.
Cited by
-
Chemoproteomic development of SLC15A4 inhibitors with anti-inflammatory activity.Nat Chem Biol. 2024 Aug;20(8):1000-1011. doi: 10.1038/s41589-023-01527-8. Epub 2024 Jan 8. Nat Chem Biol. 2024. PMID: 38191941 Free PMC article.
-
Stereocontrolled Synthesis of the Portimine Skeleton via Organocatalyst-Mediated Asymmetric Stannylation and Stereoretentive C(sp3)-C(sp2) Stille Coupling.Org Lett. 2025 Jan 31;27(4):942-947. doi: 10.1021/acs.orglett.4c04245. Epub 2025 Jan 22. Org Lett. 2025. PMID: 39838860 Free PMC article.
-
Total Synthesis of Njaoamine C by Concurrent Macrocycle Formation.J Am Chem Soc. 2023 Oct 4;145(39):21197-21202. doi: 10.1021/jacs.3c08410. Epub 2023 Sep 21. J Am Chem Soc. 2023. PMID: 37734001 Free PMC article.
-
A Comparative Study of the In Vitro Intestinal Permeability of Pinnatoxins and Portimine.Mar Drugs. 2025 Jan 7;23(1):26. doi: 10.3390/md23010026. Mar Drugs. 2025. PMID: 39852528 Free PMC article.
-
Mechanistic differences between linear vs. spirocyclic dialkyldiazirine probes for photoaffinity labeling.Chem Sci. 2024 Aug 13;15(37):15463-73. doi: 10.1039/d4sc04238g. Online ahead of print. Chem Sci. 2024. PMID: 39246352 Free PMC article.
References
-
- Selwood AI et al. Portimine: a bioactive metabolite from the benthic dinoflagellate Vulcanodinium rugosum. Tetrahedron Lett. 54, 4705–4707 (2013).
-
- Cuddihy SL et al. The marine cytotoxin portimine is a potent and selective inducer of apoptosis. Apoptosis 21, 1447–1452 (2016). - PubMed
-
- Jørgensen L. et al. 14-Step Synthesis of (+)-Ingenol from (+)-3-Carene. Science 341, 878–882 (2013). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

