Neoadjuvant botensilimab plus balstilimab response pattern in locally advanced mismatch repair proficient colorectal cancer
- PMID: 37731056
- PMCID: PMC10611560
- DOI: 10.1038/s41388-023-02835-y
Neoadjuvant botensilimab plus balstilimab response pattern in locally advanced mismatch repair proficient colorectal cancer
Abstract
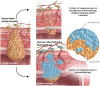
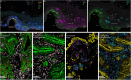
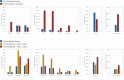
In patients with locally advanced cancer without distant metastases, the neoadjuvant setting presents a platform to evaluate new drugs. For mismatch repair proficient/microsatellite stable (pMMR/MSS) colon and rectal cancer, immunotherapy has shown limited efficacy. Herein, we report exceptional responses observed with neoadjuvant botensilimab (BOT), an Fc-enhanced next-generation anti-CTLA-4 antibody, alongside balstilimab (BAL; an anti-PD-1 antibody) in two patients with pMMR/MSS colon and rectal cancer. The histological pattern of rapid immune response observed ("inside-out" (serosa-to-mucosa) tumor regression) has not been described previously in this setting. Spatial biology analyses (RareCyte Inc.) reveal mechanisms of actions of BOT, a novel innate-adaptive immune activator. These observations have downstream implications for clinical trial designs using neoadjuvant immunotherapy and potentially sparing patients chemotherapy.
© 2023. The Author(s).
Conflict of interest statement
Author PMK reports grants paid to the institution by Merck, Agenus Bio, Novartis, Advanced Accelerator Applications, Tersera, and Boston Scientific; a consultancy and advisory board relationship with Elicio (scientific advisory board member/shares/stock ownership); consultancy/advisory board fees from Guardant Health, Natera, Foundation Medicine, Illumina, BostonGene, Merck/MSD Oncology, Tempus, Bayer, Lilly, Delcath Systems, IPBA, QED Therapeutics, Boston Healthcare Associates, Servier, Taiho Oncology, Exact Sciences, Daiichi Sankyo/AstraZeneca, Eisai, Saga Diagnostics, Neogenomics, Do More Diagnostics AS, and Seattle Genetics; consulting fees paid to the institution by Taiho Pharmaceutical and Ipsen; receiving travel support from AstraZeneca for presentation of an investigator initiated trial. All other authors report no COIs. MH is a member of the board of directors for Bristol Myers Squibb; is a founder of Champions Oncology, Inc., and Nelum Inc.; holds stock options for Champions Oncology, Inc.; InxMed; Biooncotech; and Bristol Myers Squibb; has received research support from Agenus; has received honoraria from InxMed, Oncomatrix, MiNKi, and Peaches Biotech; and has received royalties from Myriad and Khar. MDJ has received honorarium as a speaker for Covidien. A.P. has received honorarium as a consultant for Medtronic, Vioptix, Ethicon, and Intuitive. MAS reports receiving research funding from Astellas Pharma Inc., Merck, Bristol Myers Squibb and Oncolys BioPharma and serving a leadership or judiciary role in board, society, committee or advocacy groups for the American Society of Clinical Oncology Leadership Council. U.K. reports honoraria and travel/accommodations from Cardinal Health, and 2nd.MD. DS reports consultancy for Ciox Health and Zephyr AI, Inc., and speaker/lecturer for Natera, Inc. AJO discloses royalties from Guardant Health and Natera. All other authors have no declared competing interests or financial ties to disclose.
Figures


References
-
- Chalabi M. The promise of neoadjuvant immunotherapy across solid tumors. 2023. https://meetings.asco.org/2023-asco-annual-meeting/15057?presentation=22... (Accessed 18 Aug 2023).
-
- El-Khoueiry AB, Fakih M, Gordon MS, Tsimberidou AM, Bullock AJ, Wilky BA, et al. Results from a phase 1a/1b study of botensilimab (BOT), a novel innate/adaptive immune activator, plus balstilimab (BAL; anti-PD-1 antibody) in metastatic heavily pretreated microsatellite stable colorectal cancer (MSS CRC) J Clin Oncol. 2023;41:LBA8. doi: 10.1200/JCO.2023.41.4_suppl.LBA8. - DOI
-
- Delepine C, Levey D, Krishnan S, Kim K-S, Sonabend A, Wilkens M, et al. 470 Botensilimab, an Fc-enhanced CTLA-4 antibody, enhances innate and adaptive immune activation to promote superior anti-tumor immunity in cold and I-O refractory tumors. J Immunother Cancer. 2022;10:A490.

