Senescent cells at the crossroads of aging, disease, and tissue homeostasis
- PMID: 37731189
- PMCID: PMC10776127
- DOI: 10.1111/acel.13988
Senescent cells at the crossroads of aging, disease, and tissue homeostasis
Abstract
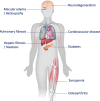
Originally identified as an outcome of continuous culture of primary cells, cellular senescence has moved beyond the culture dish and is now a bona fide driver of aging and disease in animal models, and growing links to human disease. This cellular stress response consists of a stable proliferative arrest coupled to multiple phenotypic changes. Perhaps the most important of these is the senescence-associated secretory phenotype, or senescence-associated secretory phenotype -a complex and variable collection of secreted molecules release by senescent cells with a number of potent biological activities. Senescent cells appear in multiple age-associated conditions in humans and mice, and interventions that eliminate these cells can prevent or even reverse multiple diseases in mouse models. Here, we review salient aspects of senescent cells in the context of human disease and homeostasis. Senescent cells increase in abundance during several diseases that associated with premature aging. Conversely, senescent cells have a key role in beneficial processes such as development and wound healing, and thus can help maintain tissue homeostasis. Finally, we speculate on mechanisms by which deleterious aspects of senescent cells might be targeted while retaining homeostatic aspects in order to improve age-related outcomes.
Keywords: cellular senescence; disease drivers of aging; homeostasis; progeria; senolytics.
© 2023 The Authors. Aging Cell published by Anatomical Society and John Wiley & Sons Ltd.
Conflict of interest statement
CW is an inventor on patents related to the detection, modification, and elimination of senescent cells. CK declares no conflicts of interest. The content is the sole responsibility of the authors and does not necessarily represent the official views of the USDA.
Figures


References
-
- Acosta, J. C. , Banito, A. , Wuestefeld, T. , Georgilis, A. , Janich, P. , Morton, J. P. , Athineos, D. , Kang, T. W. , Lasitschka, F. , Andrulis, M. , Pascual, G. , Morris, K. J. , Khan, S. , Jin, H. , Dharmalingam, G. , Snijders, A. P. , Carroll, T. , Capper, D. , Pritchard, C. , … Gil, J. (2013). A complex secretory program orchestrated by the inflammasome controls paracrine senescence. Nature Cell Biology, 15(8), 978–990. 10.1038/ncb2784 - DOI - PMC - PubMed
-
- Aguayo‐Mazzucato, C. , Andle, J. , Lee, T. B., Jr. , Midha, A. , Talemal, L. , Chipashvili, V. , Hollister‐Lock, J. , van Deursen, J. , Weir, G. , & Bonner‐Weir, S. (2019). Acceleration of beta cell aging determines diabetes and Senolysis improves disease outcomes. Cell Metabolism, 30(1), 129–142.e4. 10.1016/j.cmet.2019.05.006 - DOI - PMC - PubMed
-
- Amor, C. , Feucht, J. , Leibold, J. , Ho, Y. J. , Zhu, C. , Alonso‐Curbelo, D. , Mansilla‐Soto, J. , Boyer, J. A. , Li, X. , Giavridis, T. , Kulick, A. , Houlihan, S. , Peerschke, E. , Friedman, S. L. , Ponomarev, V. , Piersigilli, A. , Sadelain, M. , & Lowe, S. W. (2020). Senolytic CAR T cells reverse senescence‐associated pathologies. Nature, 583(7814), 127–132. 10.1038/s41586-020-2403-9 - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

