Idecabtagene vicleucel chimeric antigen receptor T-cell therapy for relapsed/refractory multiple myeloma with renal impairment
- PMID: 37731379
- PMCID: PMC10905101
- DOI: 10.3324/haematol.2023.283940
Idecabtagene vicleucel chimeric antigen receptor T-cell therapy for relapsed/refractory multiple myeloma with renal impairment
Abstract
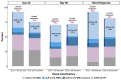
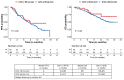
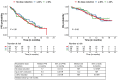
We evaluated patients with relapsed multiple myeloma with renal impairment (RI) treated with standard of care idecabtagene vicleucel (ide-cel), as outcomes with chimeric antigen receptor (CAR) T-cell therapy are unknown in this population. RI was defined as creatinine clearance (CrCl) <50 mL/min. CrCl of <30 mL/min or dialysis dependence were defined as severe RI. The study cohort included 214 patients, 28 (13%) patients with RI, including 11 patients severe RI (dialysis, N=1). Patients with RI were older, more likely to be female and had higher likelihood of having Revised International Staging System stage 3 disease. Rates and severity of cytokine release syndrome (89% vs. 84%, grade ≥3: 7% vs. 2%) and immune effector cell-associated neurotoxicity syndrome (23% vs. 20%) were similar in patients with and without RI, respectively. Patients with RI had higher incidence of short-term grade ≥3 cytopenias, although cytopenias were similar by 3 months following CAR T-cell therapy. Renal function did not worsen after CAR T-cell therapy in patients with RI. Response rates (93% vs. 82%) and survival outcomes (median progression-free survival: 9 vs. 8 months; P=0.26) were comparable in patients with and without RI, respectively. Treatment with ide-cel is feasible in patients with RI, with a comparable safety and efficacy profile as patients without RI, with notable exception of higher short-term high-grade cytopenias.
Figures
References
-
- Mikhael J. Treatment options for triple-class refractory multiple myeloma. Clin Lymphoma Myeloma Leuk. 2020;20(1):1-7. - PubMed
-
- Usmani S, Ahmadi T, Ng Y, et al. . Analysis of real-world data on overall survival in multiple myeloma patients with ≥3 prior lines of therapy including a proteasome inhibitor (PI) and an immunomodulatory drug (IMiD), or double refractory to a PI and an IMiD. Oncologist. 2016;21(11):1355-1361. - PMC - PubMed
-
- Chari A, Vogl DT, Gavriatopoulou M, et al. . Oral selinexor– dexamethasone for triple-class refractory multiple myeloma. N Engl J Med. 2019;381(8):727-738. - PubMed
-
- BMS Pharma: ABECMA (idecabtagene vicleucel)[package insert]. https://packageinserts.bms.com/pi/pi_abecma.pdf.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

