Cost-effectiveness analysis of dostarlimab plus carboplatin-paclitaxel as first-line treatment for advanced endometrial cancer
- PMID: 37731489
- PMCID: PMC10507332
- DOI: 10.3389/fimmu.2023.1267322
Cost-effectiveness analysis of dostarlimab plus carboplatin-paclitaxel as first-line treatment for advanced endometrial cancer
Abstract
Background: A recent phase III clinical trial (NCT03981796) evaluated the efficacy and safety of dostarlimab combined with carboplatin-paclitaxel (DOS-CP) compared to placebo combined with carboplatin-paclitaxel (PLB-CP) as a first-line treatment for advanced endometrial cancer (EC). The NCT03981796 trial demonstrated that DOS-CP significantly improved progression-free survival and overall survival of patients with advanced EC while maintaining an acceptable safety profile. However, DOS-CP is expensive and its cost-effectiveness has not been evaluated. This study aims to evaluate the cost-effectiveness of DOS-CP compared to PLB-CP as a first-line treatment for advanced EC from the perspective of the Chinese healthcare system.
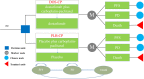
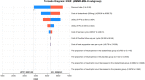
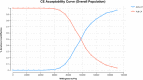
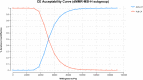
Methods: A Markov model with three health states was developed to evaluate the cost-effectiveness of DOS-CP as a first-line treatment for advanced EC. Clinical efficacy data were derived from the NCT03981796 trial, and drug costs were determined based on national tender prices. Other costs and utility values were obtained from published literature. The outcomes assessed included total costs, quality-adjusted life years (QALYs), and incremental cost-effectiveness ratios (ICERs). The robustness of the model was assessed through one-way sensitivity analysis and probabilistic sensitivity analysis.
Results: In comparison to PLB-CP, the ICER of DOS-CP was $98,276.61/QALY for the overall population, $53,063.61/QALY for the dMMR subgroup, and $124,088.56/QALY for the pMMR subgroup. All of these ICER values were higher than the willingness-to-pay threshold of $38,201 per QALY. The most important variable that affected the results of the model was the discount rate, the cost of dostarlimab, and the utility value for progressive disease.
Conclusion: From the perspective of the Chinese healthcare system, DOS-CP is unlikely to be a cost-effective first-line treatment option for advanced EC.
Keywords: carboplatin-paclitaxel; cost-effectiveness; dostarlimab; endometrial cancer; first-line treatment.
Copyright © 2023 You, Zeng, Zhang, Huang, Zhang, Cai and Hu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

