Novel engineered B lymphocytes targeting islet-specific T cells inhibit the development of type 1 diabetes in non-obese diabetic Scid mice
- PMID: 37731505
- PMCID: PMC10507356
- DOI: 10.3389/fimmu.2023.1227133
Novel engineered B lymphocytes targeting islet-specific T cells inhibit the development of type 1 diabetes in non-obese diabetic Scid mice
Abstract
Introduction: In this study, we report a novel therapeutic approach using B lymphocytes to attract islet-specific T cells in the non-obese diabetic (NOD) mouse model and prevent the development of autoimmune diabetes. Rather than using the antibody receptor of B cells, this approach utilizes their properties as antigen-presenting cells to T cells.
Methods: Purified splenic B cells were treated with lipopolysaccharide, which increases regulatory B (Breg) cell function, then electroporated with mRNA encoding either chimeric MHC-I or MHC-II molecules covalently linked to antigenic peptides. Immunoregulatory functions of these engineered B cells (e-B cells) were tested by in vitro assays and in vivo co-transfer experiments with beta-cell-antigen-specific CD8+ or CD4+ T cells in NOD.Scid mice, respectively.
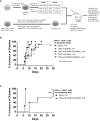
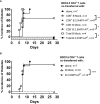
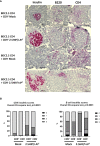
Results: The e-B cells expressing chimeric MHC-I-peptide inhibited antigen-specific CD8+ T-cell cytotoxicity in vitro. The e-B cells expressing chimeric MHC-II-peptide induced antigen-specific CD4+ T cells to express the regulatory markers, PD-1, ICOS, CTLA-4, Lag3, and Nrp1. Furthermore, e-B cells encoding the chimeric MHC-I and MHC-II peptide constructs protected NOD.Scid mice from autoimmune diabetes induced by transfer of antigen-specific CD8+ and CD4+ T cells.
Discussion: MHC-peptide chimeric e-B cells interacted with pathogenic T cells, and protected the host from autoimmune diabetes, in a mouse model. Thus, we have successfully expressed MHC-peptide constructs in B cells that selectively targeted antigen-specific cells, raising the possibility that this strategy could be used to endow different protective cell types to specifically regulate/remove pathogenic cells.
Keywords: CD4+ T cells; CD8+ T cells; NOD mice; regulatory B cells; type 1 diabetes.
Copyright © 2023 Chen, Kakabadse, Fishman, Weinstein-Marom, Davies, Boldison, Thayer, Wen, Gross and Wong.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures






References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

