CD51 labels periosteal injury-responsive osteoprogenitors
- PMID: 37731543
- PMCID: PMC10507171
- DOI: 10.3389/fphys.2023.1231352
CD51 labels periosteal injury-responsive osteoprogenitors
Abstract
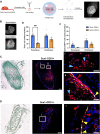
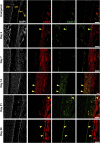
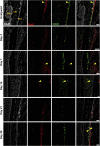
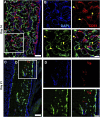
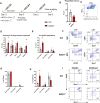
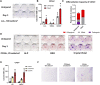
The periosteum is a critical source of skeletal stem and progenitor cells (SSPCs) that form callus tissue in response to injury. There is yet to be a consensus on how to identify SSPCs in the adult periosteum. The aim of this study was to understand how potential murine periosteal SSPC populations behave in vivo and in response to injury. We evaluated the in vivo differentiation potential of Sca1-CD51+ and Sca1+CD51+ cells following transplantation. In vitro, the Sca1+CD51+ population appears to be more primitive multipotent cells, but after transplantation, Sca1-CD51+ cells showed superior engraftment, expansion, and differentiation into chondrocytes and osteoblasts. Despite representing a clear population with flow cytometry, we identified very few Sca1+CD51+ cells histologically. Using a periosteal scratch injury model, we successfully mimicked the endochondral-like healing process seen in unstable fractures, including the expansion and osteochondral differentiation of αSMA+ cells following injury. CD51+ cells were present in the cambium layer of resting periosteum and expanded following injury. Sca1+CD51- cells were mainly localized in the outer periosteal layer. We found that injury increased colony-forming unit fibroblast (CFU-F) formation in the periosteum and led to rapid expansion of CD90+ cells. Several other populations, including Sca1-CD51+ and CD34+ cells, were expanded by day 7. Mice with enhanced fracture healing due to elevated Notch signaling mediated by NICD1 overexpression showed significant expansion of CD51+ and CD34hi cells in the early stages of healing, suggesting these populations contribute to more rapid healing. In conclusion, we demonstrate that periosteal injury leads to the expansion of various SSPC populations, but further studies are required to confirm their lineage hierarchy in the adult skeletal system. Our data indicate that CD51+ skeletal progenitor cells are injury-responsive and show good engraftment and differentiation potential upon transplantation.
Keywords: CD34; CD51; Notch; SCA1; fracture; periosteum.
Copyright © 2023 Cao, Kalajzic and Matthews.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Cao Y., Bolam S. M., Boss A. L., Murray H. C., Dalbeth N., Brooks A. E. S., et al. (2022). Characterization of adult human skeletal cells in different tissues reveals a CD90+CD34+ periosteal stem cell population. Cold Spring Harbor Laboratory. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

