Neoadjuvant chemoimmunotherapy cycle number selection for non-small cell lung cancer and clinical outcomes: a real-world analysis
- PMID: 37731645
- PMCID: PMC10508959
- DOI: 10.3389/fonc.2023.1200625
Neoadjuvant chemoimmunotherapy cycle number selection for non-small cell lung cancer and clinical outcomes: a real-world analysis
Abstract
Objectives: Neoadjuvant chemoimmunotherapy is the optimal choice in the treatment of NSCLC; however, the optimal number of therapeutic cycles remains unclear. The primary aim of this study was to determine the optimal number of neoadjuvant therapeutic cycles in NSCLC.
Methods: This study was a real-world clinical analysis that included patients who received neoadjuvant chemoimmunotherapy followed by surgery from January 2020 to August 2022. Patients were divided into two groups based on the number of therapeutic cycles: 2-cycle group and 3-4-cycles group. The primary endpoint was the major pathological response (MPR) rate.
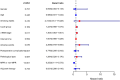
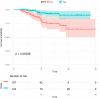
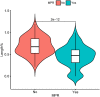
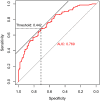
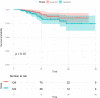
Results: A total of 251 patients were included: 150 in the 2-cycle group and 101 in the 3-4-cycles group. Baseline characteristics were well-balanced between the groups. The MPR in the 2-cycle group was 57.3% and not significantly different from that of 57.4% in the 3-4-cycles group (p=0.529). Thirty-two patients (31.7%) in the 3-4-cycles group underwent surgery > 42 days after the final cycle of neoadjuvant therapy, significantly more than the 24 patients (16.0%) in the 2-cycle group (p=0.003). The incidence of adverse events related to neoadjuvant therapy was higher in the 3-4-cycles vs 2-cycle groups (72.3% versus 58.0%, respectively; p=0.021), while the 2-cycle group had a higher rate of postoperative morbidities (28.0% versus 12.9%, respectively; p=0.004). Additionally, for patients with ≤ 44.2% regression in diameter on computed tomography after two cycles of treatment, the MPR rate was higher in the 3-4-cycles vs 2-cycle group (47.3% versus 29.9%, respectively; p=0.048). For cases with programmed death-ligand 1 expression, regarding tumor proportion score ≤ 10%, 3-4 cycles of neoadjuvant treatment increased the MPR rate compared with 2 cycles (37.5% versus 9.5%, respectively; p=0.041).
Conclusion: Our data support the positive role of chemoimmunotherapy in the neoadjuvant treatment of NSCLC. Extending to 3-4 cycles instead of 2 cycles of neoadjuvant chemoimmunotherapy may improve the safety of surgery and result in a lower incidence of postoperative morbidities; however, the MPR rate may not increase significantly. CT re-evaluation during treatment and PD-L1 expression at initial diagnosis are potential indicators to guide the choice of the number of therapeutic cycles.
Keywords: adverse events; morbidity; neoadjuvant chemoimmunotherapy; non-small cell lung cancer; treatment cycles.
Copyright © 2023 Zhang, Guo, Jia, Wang, Wu, Chen, Li, Yang, Li, Wang and Xiao.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Provencio M, Serna-Blasco R, Nadal E, Insa A, García-Campelo MR, Casal Rubio J, et al. Overall Survival and Biomarker Analysis of Neoadjuvant Nivolumab Plus Chemotherapy in Operable Stage IIIA Non-Small-Cell Lung Cancer (NADIM phase II trial). J Clin Oncol (2022) 40(25):2924–33. doi: 10.1200/JCO.21.02660 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

