Restoring susceptibility to aminoglycosides: identifying small molecule inhibitors of enzymatic inactivation
- PMID: 37731693
- PMCID: PMC10507813
- DOI: 10.1039/d3md00226h
Restoring susceptibility to aminoglycosides: identifying small molecule inhibitors of enzymatic inactivation
Abstract
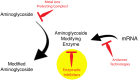
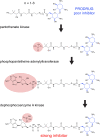
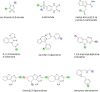
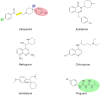
Growing resistance to antimicrobial medicines is a critical health problem that must be urgently addressed. Adding to the increasing number of patients that succumb to infections, there are other consequences to the rise in resistance like the compromise of several medical procedures and dental work that are heavily dependent on infection prevention. Since their introduction in the clinics, aminoglycoside antibiotics have been a critical component of the armamentarium to treat infections. Still, the increase in resistance and their side effects led to a decline in their utilization. However, numerous current factors, like the urgent need for antimicrobials and their favorable properties, led to renewed interest in these drugs. While efforts to design new classes of aminoglycosides refractory to resistance mechanisms and with fewer toxic effects are starting to yield new promising molecules, extending the useful life of those already in use is essential. For this, numerous research projects are underway to counter resistance from different angles, like inhibition of expression or activity of resistance components. This review focuses on selected examples of one aspect of this quest, the design or identification of small molecule inhibitors of resistance caused by enzymatic modification of the aminoglycoside. These compounds could be developed as aminoglycoside adjuvants to overcome resistant infections.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
None to declare.
Figures

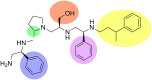
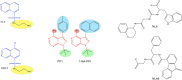
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

