What makes joint assessment procedures attractive to the innovative industry: successes, challenges, and proposed improvements
- PMID: 37731723
- PMCID: PMC10507468
- DOI: 10.3389/fmed.2023.1207954
What makes joint assessment procedures attractive to the innovative industry: successes, challenges, and proposed improvements
Abstract
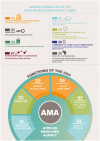
Regulatory harmonization and convergence have been identified as the key driver in promoting efficient evaluation of medicines, reducing workload, and supporting earlier access to medicines on the African continent. There has been great progress to date in enhancing regulatory harmonization and convergence on the African continent via the Regional Economic Communities (RECs) and with the establishment of the Africa Medicines Agency (AMA). In this article, the International Federation of Pharmaceutical Manufacturers and Associations (IFPMA) Africa Regulatory Network (ARN) presents its perspective based on the available literature review and results from a survey conducted with innovative biopharmaceutical companies to gather experiences using regional joint assessment procedures (JAPs) in Africa, such as the East African Community Medicines Regulatory Harmonization (EAC-MRH), the West African Medicines Regulatory Harmonization (WA-MRH), and the Southern African Development Community Medicines Regulatory Harmonization (SADC-MRH) initiative through the ZAZIBONA Collaborative Procedure for Medicines Registration (ZaZiBoNa), and provides best practices in this evolving landscape. The article also assesses other collaborative registration pathways available to facilitating registration of pharmaceutical products in African countries, such as WHO Collaborative Registration Procedures (CRP), Swissmedic's Marketing Authorisation for Global Health Products (MAGHP) and EU Medicines for All (EU-M4ALL). Benefits and challenges of each of the existing pathways are discussed in this article. Main benefits include building more expert capacity and improved collaboration amongst experts, as well as shorter review timelines in some cases. Key challenges include the lack of predictability in the adherence to procedural timelines as defined per guidelines, lengthy timeline to achieve national marketing authorization following joint assessment, the lack of dedicated personnel, administrative issues during the submission process as well as additional country-specific requirements on top of JAP-specific requirements. Our recommendations for improvements include harmonization of requirements across countries and regions and with international standards, appropriate resource allocation for JAP activities to ensure adherence to timelines, use of JAPs throughout the entire product lifecycle and all product categories, adequate use of digital technologies, and improved communication and transparency with applicants. These improvements will allow industry to better plan their filing strategies for the region which will lead to overall improved usability of the JAPs in Africa and enable faster patient access.
Keywords: African Medicines Agency; African Medicines Regulatory Harmonization; collaboration; joint assessment procedures; regulatory agilities; regulatory convergence; reliance.
Copyright © 2023 Miletic, Adam, Acquah, Aziz, Joos and Mwangi.
Conflict of interest statement
NM is employed by F. Hoffmann-La Roche. JA is employed by Johnson & Johnson. JM is employed by Bayer Pharmaceuticals. ZA is employed by Novartis. AJ is employed by MSD. The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




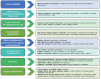
References
-
- African Union . Treaty for the establishment of the African medicines agency. Available at: https://au.int/sites/default/files/treaties/36892-treaty-0069_-_ama_trea... (Accessed February 20, 2023).
-
- United Nation Population Fund . (2012). UN commission on life-saving commodities for women and children. Available at: https://www.unfpa.org/publications/un-commission-life-saving-commodities...
-
- East African Community . (2022). EAC medicines regulatory guidelines. Available at: https://www.eac.int/medicines-regulatory-guidelines (Accessed November 2, 2022).
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

