A quick algorithmic review on management of viral infectious diseases in pediatric solid organ transplant recipients
- PMID: 37732007
- PMCID: PMC10507262
- DOI: 10.3389/fped.2023.1252495
A quick algorithmic review on management of viral infectious diseases in pediatric solid organ transplant recipients
Abstract
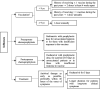
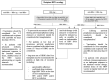
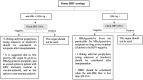
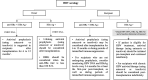
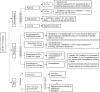
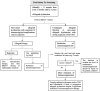
Pediatric solid organ transplant is a life-saving procedure for children with end-stage organ failure. Viral infections are a common complication following pediatric solid organ transplantation (SOT), which can lead to increased morbidity and mortality. Pediatric solid organ transplant recipients are at an increased risk of viral infections due to their immunosuppressed state. The most commonly encountered viruses include cytomegalovirus (CMV), Epstein-Barr virus (EBV), herpes simplex virus (HSV), varicella-zoster virus (VZV), adenoviruses, and BK polyomavirus. Prevention strategies include vaccination prior to transplantation, post-transplant prophylaxis with antiviral agents, and preemptive therapy. Treatment options vary depending on the virus and may include antiviral therapy and sometimes immunosuppression modification. This review provides a Quick Algorithmic overview of prevention and treatment strategies for viral infectious diseases in pediatric solid organ transplant recipient.
Keywords: antiviral; pediatrics; solid organ; transplantation; viral infections.
© 2023 Moghadamnia, Eshaghi, Alimadadi and Dashti-Khavidaki.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
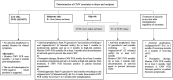
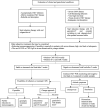

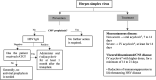
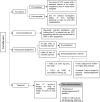
References
Publication types
LinkOut - more resources
Full Text Sources

