Immunomodulatory biomaterials against bacterial infections: Progress, challenges, and future perspectives
- PMID: 37732016
- PMCID: PMC10507240
- DOI: 10.1016/j.xinn.2023.100503
Immunomodulatory biomaterials against bacterial infections: Progress, challenges, and future perspectives
Abstract
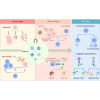
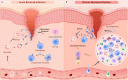
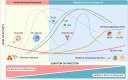
Bacterial infectious diseases are one of the leading causes of death worldwide. Even with the use of multiple antibiotic treatment strategies, 4.95 million people died from drug-resistant bacterial infections in 2019. By 2050, the number of deaths will reach 10 million annually. The increasing mortality may be partly due to bacterial heterogeneity in the infection microenvironment, such as drug-resistant bacteria, biofilms, persister cells, intracellular bacteria, and small colony variants. In addition, the complexity of the immune microenvironment at different stages of infection makes biomaterials with direct antimicrobial activity unsatisfactory for the long-term treatment of chronic bacterial infections. The increasing mortality may be partly attributed to the biomaterials failing to modulate the active antimicrobial action of immune cells. Therefore, there is an urgent need for effective alternatives to treat bacterial infections. Accordingly, the development of immunomodulatory antimicrobial biomaterials has recently received considerable interest; however, a comprehensive review of their research progress is lacking. In this review, we focus mainly on the research progress and future perspectives of immunomodulatory antimicrobial biomaterials used at different stages of infection. First, we describe the characteristics of the immune microenvironment in the acute and chronic phases of bacterial infections. Then, we highlight the immunomodulatory strategies for antimicrobial biomaterials at different stages of infection and their corresponding advantages and disadvantages. Moreover, we discuss biomaterial-mediated bacterial vaccines' potential applications and challenges for activating innate and adaptive immune memory. This review will serve as a reference for future studies to develop next-generation immunomodulatory biomaterials and accelerate their translation into clinical practice.
© 2023.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Stewart P.S., Bjarnsholt T. Risk factors for chronic biofilm-related infection associated with implanted medical devices. Clin. Microbiol. Infect. 2020;26:1034–1038. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

