Clinical Manifestations and Risk Factors of Tigecycline-Associated Thrombocytopenia
- PMID: 37732172
- PMCID: PMC10508280
- DOI: 10.2147/IDR.S426259
Clinical Manifestations and Risk Factors of Tigecycline-Associated Thrombocytopenia
Abstract
Background: Thrombocytopenia, characterized by a diminished platelet count, emerged as the most frequently reported coagulation dysfunction event according to the FDA Adverse Event Reporting System (FAERS) database. In recent years, numerous clinical studies have investigated the potential link between tigecycline usage and the occurrence of hypofibrinogenemia. However, a research gap remains in comprehensively examining the association between tigecycline and thrombocytopenia in real-world settings.
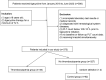
Methods: This study was conducted to explore the incidence and clinical manifestations of tigecycline-associated thrombocytopenia. A retrospective case-control study of patients treated with tigecycline was conducted between January 2018 and June 2022.
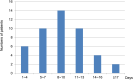
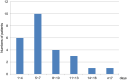
Results: In total, 373 patients were included in this study. Among these patients, 12.3% experienced thrombocytopenia. The onset of thrombocytopenia occurred within a range of 2 to 22 days after the initiation of tigecycline, with a median period (25-75th percentile) of 9 (6-11) days. Among the patients manifesting thrombocytopenia, 60.9% exhibited mild-to-moderate cases (grades 1-2) while 39.1% endured severe cases (grades 3-4). Multivariate analysis delineated several factors as independent risk factors for thrombocytopenia. Notably, advanced age (≥74 years) (p=0.028), risk of malnutrition (p<0.001), tigecycline therapy for ≥7 days (p=0.003), DBIL>8.1μmol/L (p<0.001)), BUN>8.1mmol/L (p=0.002) emerged as independent risk factors associated with thrombocytopenia. When comparing the control group to the thrombocytopenia group, 70.7% of patients in the control group exhibited 0-2 risk factors, while all patients in the thrombocytopenia group demonstrated risk factors. Specifically, 95.7% of patients in the thrombocytopenia group presented with three to five risk factors, with only 4.4% having 0-2 risk factors.
Conclusion: Tigecycline administration is associated with thrombocytopenia. Healthcare professionals should exercise vigilance, particularly in cases of severe tigecycline-associated thrombocytopenia, and undertake routine monitoring of patients' platelet counts, especially for those who possess three or more risk factors.
Keywords: coagulation disorder; pharmacovigilance; risk factor; thrombocytopenia; tigecycline.
© 2023 Zhu et al.
Conflict of interest statement
The authors declare the absence of known competing financial interests or personal relationships that could potentially influence the work reported in this paper.
Figures
Similar articles
-
Coagulation dysfunction events associated with tigecycline: a real-world study from FDA adverse event reporting system (FAERS) database.Thromb J. 2022 Mar 5;20(1):12. doi: 10.1186/s12959-022-00369-z. Thromb J. 2022. PMID: 35248072 Free PMC article.
-
Identification of Risk Factors and Predictive Indicators for Tigecycline-Associated Hypofibrinogenemia.Clin Transl Sci. 2025 Apr;18(4):e70213. doi: 10.1111/cts.70213. Clin Transl Sci. 2025. PMID: 40181433 Free PMC article.
-
Incidence, characteristics and risk factors of hypofibrinogenemia associated with tigecycline: A multicenter retrospective study in China.Front Pharmacol. 2022 Oct 11;13:943674. doi: 10.3389/fphar.2022.943674. eCollection 2022. Front Pharmacol. 2022. PMID: 36304151 Free PMC article.
-
Severe Coagulation Disorder and Thrombocytopenia Associated with Tigecycline - Case Report and Review of Literature.Curr Drug Saf. 2017;12(1):7-9. doi: 10.2174/1574886311666160920090714. Curr Drug Saf. 2017. PMID: 27659942 Review.
-
Incidence, characteristics, and risk factors of hypofibrinogenemia induced by generic tigecycline: a retrospective study.Naunyn Schmiedebergs Arch Pharmacol. 2025 Mar;398(3):2717-2727. doi: 10.1007/s00210-024-03419-7. Epub 2024 Sep 10. Naunyn Schmiedebergs Arch Pharmacol. 2025. PMID: 39254879 Free PMC article. Review.
Cited by
-
Incidence and risk factors of Vancomycin-induced thrombocytopenia: a six-year real-world study.BMC Infect Dis. 2025 Jan 2;25(1):7. doi: 10.1186/s12879-024-10393-1. BMC Infect Dis. 2025. PMID: 39748286 Free PMC article.
-
Third-Generation Tetracyclines: Current Knowledge and Therapeutic Potential.Biomolecules. 2024 Jun 30;14(7):783. doi: 10.3390/biom14070783. Biomolecules. 2024. PMID: 39062497 Free PMC article. Review.
-
Molecular Analysis of Tigecycline Resistance in Carbapenem-Resistant Enterobacterales (CRE) in Mthatha and Surrounding Hospitals.Antibiotics (Basel). 2025 Apr 16;14(4):407. doi: 10.3390/antibiotics14040407. Antibiotics (Basel). 2025. PMID: 40298581 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Miscellaneous