This is a preprint.
Anatomical Modeling and Optimization of Speckle Contrast Optical Tomography
- PMID: 37732196
- PMCID: PMC10508753
- DOI: 10.1101/2023.09.06.556565
Anatomical Modeling and Optimization of Speckle Contrast Optical Tomography
Abstract
Traditional methods for mapping cerebral blood flow (CBF), such as positron emission tomography and magnetic resonance imaging, offer only isolated snapshots of CBF due to scanner logistics. Speckle contrast optical tomography (SCOT) is a promising optical technique for mapping CBF. However, while SCOT has been established in mice, the method has not yet been demonstrated in humans - partly due to a lack of anatomical reconstruction methods and uncertainty over the optimal design parameters. Herein we develop SCOT reconstruction methods that leverage MRI-based anatomical head models and finite-element modeling of the SCOT forward problem (NIRFASTer). We then simulate SCOT for CBF perturbations to evaluate sensitivity of imaging performance to exposure time and SD-distances. We find image resolution comparable to intensity-based diffuse optical tomography at superficial cortical tissue depth (~1.5 cm). Localization errors can be reduced by including longer SD-measurements. With longer exposure times speckle contrast decreases, however, noise decreases faster, resulting in a net increase in SNR. Specifically, extending exposure time from 10μs to 10ms increased SCOT SNR by 1000X. Overall, our modeling methods provide anatomically-based image reconstructions that can be used to evaluate a broad range of tissue conditions, measurement parameters, and noise sources and inform SCOT system design.
Keywords: Brain imaging; cerebral blood flow; cortical mapping; laser speckle contrast tomography; mathematical modeling.
Conflict of interest statement
8.Declaration of conflicting interests The Author(s) declare(s) that there is no conflict of interest
Figures
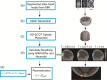

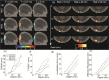


References
-
- Fan AP, Jahanian H, Holdsworth SJ, et al. Comparison of cerebral blood flow measurement with [15O]-water positron emission tomography and arterial spin labeling magnetic resonance imaging: A systematic review. J Cereb Blood Flow Metab Off J Int Soc Cereb Blood Flow Metab 2016; 36: 842–861. - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
