This is a preprint.
The Egg-Counter: A novel microfluidic platform for characterization of Caenorhabditis elegans egg-laying
- PMID: 37732270
- PMCID: PMC10508723
- DOI: 10.1101/2023.09.01.555781
The Egg-Counter: A novel microfluidic platform for characterization of Caenorhabditis elegans egg-laying
Update in
-
The egg-counter: a novel microfluidic platform for characterization of Caenorhabditis elegans egg-laying.Lab Chip. 2024 May 28;24(11):2975-2986. doi: 10.1039/d3lc01073b. Lab Chip. 2024. PMID: 38738514 Free PMC article.
Abstract
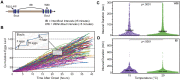
Reproduction is a fundamental process that shapes the demography of every living organism yet is often difficult to assess with high precision in animals that produce large numbers of offspring. Here, we present a novel microfluidic research platform for studying Caenorhabditis elegans' egg-laying. The platform provides higher throughput than traditional solid-media assays while providing a very high degree of temporal resolution. Additionally, the environmental control enabled by microfluidic animal husbandry allows for experimental perturbations difficult to achieve with solid-media assays. We demonstrate the platform's utility by characterizing C. elegans egg-laying behavior at two commonly used temperatures, 15 and 20°C. As expected, we observed a delayed onset of egg-laying at 15°C degrees, consistent with published temperature effects on development rate. Additionally, as seen in solid media studies, egg laying output was higher under the canonical 20°C conditions. While we validated the Egg-Counter with a study of temperature effects in wild-type animals, the platform is highly adaptable to any nematode egg-laying research where throughput or environmental control needs to be maximized without sacrificing temporal resolution.
Conflict of interest statement
Conflicts of interest The authors have no conflicts to declare.
Figures




References
-
- Carey J. R. & Roach D. Biodemography: An Introduction to Concepts and Methods. (Princeton University Press, 2020).
-
- Cury K. M., Prud’homme B. & Gompel N. A short guide to insect oviposition: when, where and how to lay an egg. J. Neurogenet. 33, 75–89 (2019). - PubMed
-
- Dweck H. K. M. et al. Olfactory preference for egg laying on citrus substrates in Drosophila. Curr. Biol. 23, 2472–2480 (2013). - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
