Combined Treatment of Human Induced Pluripotent Stem Cell-Derived Cardiomyocytes and Endothelial Cells Regenerate the Infarcted Heart in Mice and Non-Human Primates
- PMID: 37732466
- PMCID: PMC10683868
- DOI: 10.1161/CIRCULATIONAHA.122.061736
Combined Treatment of Human Induced Pluripotent Stem Cell-Derived Cardiomyocytes and Endothelial Cells Regenerate the Infarcted Heart in Mice and Non-Human Primates
Abstract
Background: Remuscularization of the mammalian heart can be achieved after cell transplantation of human induced pluripotent stem cell (hiPSC)-derived cardiomyocytes (CMs). However, several hurdles remain before implementation into clinical practice. Poor survival of the implanted cells is related to insufficient vascularization, and the potential for fatal arrhythmogenesis is associated with the fetal cell-like nature of immature CMs.
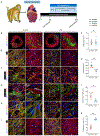
Methods: We generated 3 lines of hiPSC-derived endothelial cells (ECs) and hiPSC-CMs from 3 independent donors and tested hiPSC-CM sarcomeric length, gap junction protein, and calcium-handling ability in coculture with ECs. Next, we examined the therapeutic effect of the cotransplantation of hiPSC-ECs and hiPSC-CMs in nonobese diabetic-severe combined immunodeficiency (NOD-SCID) mice undergoing myocardial infarction (n≥4). Cardiac function was assessed by echocardiography, whereas arrhythmic events were recorded using 3-lead ECGs. We further used healthy non-human primates (n=4) with cell injection to study the cell engraftment, maturation, and integration of transplanted hiPSC-CMs, alone or along with hiPSC-ECs, by histological analysis. Last, we tested the cell therapy in ischemic reperfusion injury in non-human primates (n=4, 3, and 4 for EC+CM, CM, and control, respectively). Cardiac function was evaluated by echocardiography and cardiac MRI, whereas arrhythmic events were monitored by telemetric ECG recorders. Cell engraftment, angiogenesis, and host-graft integration of human grafts were also investigated.
Results: We demonstrated that human iPSC-ECs promote the maturity and function of hiPSC-CMs in vitro and in vivo. When cocultured with ECs, CMs showed more mature phenotypes in cellular structure and function. In the mouse model, cotransplantation augmented the EC-accompanied vascularization in the grafts, promoted the maturity of CMs at the infarct area, and improved cardiac function after myocardial infarction. Furthermore, in non-human primates, transplantation of ECs and CMs significantly enhanced graft size and vasculature and improved cardiac function after ischemic reperfusion.
Conclusions: These results demonstrate the synergistic effect of combining iPSC-derived ECs and CMs for therapy in the postmyocardial infarction heart, enabling a promising strategy toward clinical translation.
Keywords: arrhythmias, cardiac; guided tissue regeneration; induced pluripotent stem cell; models, animal.
Conflict of interest statement
Figures
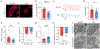




References
-
- Laflamme MA, Chen KY, Naumova AV, Muskheli V, Fugate JA, Dupras SK, Reinecke H, Xu C, Hassanipour M, Police S, et al. Cardiomyocytes derived from human embryonic stem cells in pro-survival factors enhance function of infarcted rat hearts. Nat Biotechnol. 2007;25:1015–1024. doi: 10.1038/nbt1327 - DOI - PubMed
-
- Romagnuolo R, Masoudpour H, Porta-Sánchez A, Qiang B, Barry J, Laskary A, Qi X, Massé S, Magtibay K, Kawajiri H, et al. Human Embryonic Stem Cell-Derived Cardiomyocytes Regenerate the Infarcted Pig Heart but Induce Ventricular Tachyarrhythmias. Stem Cell Reports. 2019;12:967–981. doi: 10.1016/j.stemcr.2019.04.005 - DOI - PMC - PubMed
-
- Liu Y-W, Chen B, Yang X, Fugate JA, Kalucki FA, Futakuchi-Tsuchida A, Couture L, Vogel KW, Astley CA, Baldessari A, et al. Human embryonic stem cell–derived cardiomyocytes restore function in infarcted hearts of non-human primates. Nature Biotechnology. 2018;36:597–605. doi: 10.1038/nbt.4162 - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

