A review and meta-analysis of stem cell therapies in stroke patients: effectiveness and safety evaluation
- PMID: 37733251
- PMCID: PMC10761518
- DOI: 10.1007/s10072-023-07032-z
A review and meta-analysis of stem cell therapies in stroke patients: effectiveness and safety evaluation
Abstract
Purpose: Stem cells have been extensively used during the last decade to improve clinical outcomes after stroke. The dramatic increase in trials in this field has led us to perform a systematic review and meta-analysis to understand the safety, effectiveness, and relative limitations of this type of intervention.
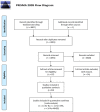
Method: This review summarizes the current evidence pooled from PubMed (Medline), EMBASE, EBSCOhost, http://clinicaltrials.gov , Scopus (Elsevier), Cochrane Central Register of Controlled Trials (CENTRAL), and Web of Science (Science Citation Index Expanded) databases for the use of stem cell therapies in stroke patients without combinations with other treatment modalities. The National Institutes of Health Stroke, modified Rankin Scales, and Barthel Index scores after external stem cell administration have been evaluated on the 3rd, 6th, and 12th months after treatment. The random effect analysis was performed using the Review Manager 5.4.1. The characteristics of stem cell sources and their adverse effects have been discussed as well.
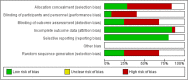
Findings: Although reasonably safe, the effectiveness evidence fluctuated to a large extent due to the heterogeneity of the clinical trials and the absence of a systematic approach. The stem cell sources and the administration window were not strongly associated with clinical outcomes.
Conclusion: Further studies should be conducted to understand the deep discrepancy between preclinical and clinical trials and to execute phase 3 clinical trials with robust control of study characteristics and outcomes.
Keywords: BI; NIHSS; Stem cell; Stroke; mRS.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
-
What is the value of routinely testing full blood count, electrolytes and urea, and pulmonary function tests before elective surgery in patients with no apparent clinical indication and in subgroups of patients with common comorbidities: a systematic review of the clinical and cost-effective literature.Health Technol Assess. 2012 Dec;16(50):i-xvi, 1-159. doi: 10.3310/hta16500. Health Technol Assess. 2012. PMID: 23302507 Free PMC article.
-
Treatments for seizures in catamenial (menstrual-related) epilepsy.Cochrane Database Syst Rev. 2021 Sep 16;9(9):CD013225. doi: 10.1002/14651858.CD013225.pub3. Cochrane Database Syst Rev. 2021. PMID: 34528245 Free PMC article.
-
Antidepressants for pain management in adults with chronic pain: a network meta-analysis.Health Technol Assess. 2024 Oct;28(62):1-155. doi: 10.3310/MKRT2948. Health Technol Assess. 2024. PMID: 39367772 Free PMC article.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2017 Dec 22;12(12):CD011535. doi: 10.1002/14651858.CD011535.pub2. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2020 Jan 9;1:CD011535. doi: 10.1002/14651858.CD011535.pub3. PMID: 29271481 Free PMC article. Updated.
Cited by
-
Preclinical safety and efficacy evaluation of the intrathecal transplantation of GMP-grade human umbilical cord mesenchymal stem cells for ischemic stroke.Neural Regen Res. 2026 Mar 1;21(3):1172-1182. doi: 10.4103/NRR.NRR-D-24-00683. Epub 2024 Nov 13. Neural Regen Res. 2026. PMID: 39589163 Free PMC article.
-
Stem Cell Therapy Modulates Molecular Cues of Vasogenic Edema Following Ischemic Stroke: Role of Sirtuin-1 in Regulating Aquaporin-4 Expression.Stem Cell Rev Rep. 2025 Apr;21(3):797-815. doi: 10.1007/s12015-025-10846-3. Epub 2025 Jan 31. Stem Cell Rev Rep. 2025. PMID: 39888572
-
Efficacy and safety of stem cell therapy for acute and subacute ischemic stroke: a systematic review and meta-analysis.Sci Rep. 2025 Jul 1;15(1):21214. doi: 10.1038/s41598-025-04405-6. Sci Rep. 2025. PMID: 40595869 Free PMC article.
-
Safety and Efficacy of Stem Cell Therapy in Ischemic Stroke: A Comprehensive Systematic Review and Meta-Analysis.J Clin Med. 2025 Mar 20;14(6):2118. doi: 10.3390/jcm14062118. J Clin Med. 2025. PMID: 40142929 Free PMC article. Review.
References
-
- Savitz SI, Yavagal D, Rappard G, et al. A phase 2 randomized, sham-controlled trial of internal carotid artery infusion of autologous bone marrow-derived ALD-401 cells in patients with recent stable ischemic stroke (RECOVER-Stroke) Circulation. 2019;139:192–205. doi: 10.1161/CIRCULATIONAHA.117.030659. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

