Peptide immunization against the C-terminal of alpha-synuclein reduces locomotor activity in mice overexpressing alpha-synuclein
- PMID: 37733672
- PMCID: PMC10513202
- DOI: 10.1371/journal.pone.0291927
Peptide immunization against the C-terminal of alpha-synuclein reduces locomotor activity in mice overexpressing alpha-synuclein
Abstract
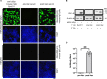
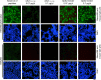
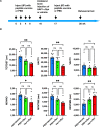
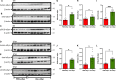
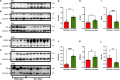
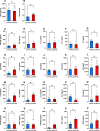
Abnormal accumulation of alpha-synuclein (αSyn) in the remaining nigra dopaminergic neurons is a common neuropathological feature found in patients with Parkinson's disease (PD). Antibody-based immunotherapy has been considered a potential approach for PD treatment. This study aims to investigate the effectiveness of active immunization against αSyn in a mouse model of PD. Adult mice were immunized with or without a synthetic peptide containing the C-terminal residues of human αSyn and activation epitopes, followed by an intranigral injection of adeno-associated virus vectors for overexpressing human αSyn. Upon the peptide injection, αSyn-specific antibodies were raised, accompanied by degeneration of dopaminergic neurons and motor deficits. Furthermore, the induction of neuroinflammation was postulated by the elevation of astroglial and microglial markers in the immunized mice. Instead of lessening αSyn toxicity, this peptide vaccine caused an increase in the pathogenic species of αSyn. Our data demonstrated the potential adverse effects of active immunization to raise antibodies against the C-terminal fragment of αSyn. This drawback highlights the need for further investigation to weigh the pros and cons of immunotherapy in PD. Applying the αSyn C-terminal peptide vaccine for PD treatment should be cautiously exercised. This study provides valuable insights into the intricate interplay among immune intervention, αSyn accumulation, and neurodegeneration.
Copyright: © 2023 Chiu et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


Similar articles
-
Administration of AAV-Alpha Synuclein NAC Antibody Improves Locomotor Behavior in Rats Overexpressing Alpha Synuclein.Genes (Basel). 2021 Jun 21;12(6):948. doi: 10.3390/genes12060948. Genes (Basel). 2021. PMID: 34205689 Free PMC article.
-
The MHC class II transactivator modulates seeded alpha-synuclein pathology and dopaminergic neurodegeneration in an in vivo rat model of Parkinson's disease.Brain Behav Immun. 2021 Jan;91:369-382. doi: 10.1016/j.bbi.2020.10.017. Epub 2020 Oct 22. Brain Behav Immun. 2021. PMID: 33223048
-
Quantitative systems pharmacology model of α-synuclein pathology in Parkinson's disease-like mouse for investigation of passive immunotherapy mechanisms.CPT Pharmacometrics Syst Pharmacol. 2024 Oct;13(10):1798-1809. doi: 10.1002/psp4.13223. Epub 2024 Aug 23. CPT Pharmacometrics Syst Pharmacol. 2024. PMID: 39177164 Free PMC article.
-
Gangliosides, α-Synuclein, and Parkinson's Disease.Prog Mol Biol Transl Sci. 2018;156:435-454. doi: 10.1016/bs.pmbts.2017.12.009. Epub 2018 Feb 24. Prog Mol Biol Transl Sci. 2018. PMID: 29747823 Review.
-
Neuropathology of α-synuclein in Parkinson's disease.Neuropathology. 2022 Apr;42(2):93-103. doi: 10.1111/neup.12812. Epub 2022 Mar 31. Neuropathology. 2022. PMID: 35362115 Review.
Cited by
-
Transplantation of Exosomes Derived From Human Wharton's Jelly Mesenchymal Stromal Cells Enhances Functional Improvement in Stroke Rats.Cell Transplant. 2024 Jan-Dec;33:9636897241296366. doi: 10.1177/09636897241296366. Cell Transplant. 2024. PMID: 39624898 Free PMC article.
-
Human serum-derived α-synuclein auto-antibodies mediate NMDA receptor-dependent degeneration of CNS neurons.J Neuroinflammation. 2024 Feb 28;21(1):62. doi: 10.1186/s12974-024-03050-6. J Neuroinflammation. 2024. PMID: 38419079 Free PMC article.
References
-
- Kahle PJ, Neumann M, Ozmen L, Muller V, Jacobsen H, Schindzielorz A, et al.. Subcellular localization of wild-type and Parkinson’s disease-associated mutant alpha -synuclein in human and transgenic mouse brain. J Neurosci. 2000;20(17):6365–73. Epub 2000/08/31. doi: 10.1523/JNEUROSCI.20-17-06365.2000 ; PubMed Central PMCID: PMC6772969. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

