Peptidergic modulation of motor neuron output via CART signaling at C bouton synapses
- PMID: 37733738
- PMCID: PMC10523464
- DOI: 10.1073/pnas.2300348120
Peptidergic modulation of motor neuron output via CART signaling at C bouton synapses
Abstract
The intensity of muscle contraction, and therefore movement vigor, needs to be adaptable to enable complex motor behaviors. This can be achieved by adjusting the properties of motor neurons, which form the final common pathway for all motor output from the central nervous system. Here, we identify roles for a neuropeptide, cocaine- and amphetamine-regulated transcript (CART), in the control of movement vigor. We reveal distinct but parallel mechanisms by which CART and acetylcholine, both released at C bouton synapses on motor neurons, selectively amplify the output of subtypes of motor neurons that are recruited during intense movement. We find that mice with broad genetic deletion of CART or selective elimination of acetylcholine from C boutons exhibit deficits in behavioral tasks that require higher levels of motor output. Overall, these data uncover spinal modulatory mechanisms that control movement vigor to support movements that require a high degree of muscle force.
Keywords: C boutons; acetylcholine; cocaine and amphetamine regulated transcript; motor control; spinal cord.
Conflict of interest statement
The authors declare no competing interest.
Figures

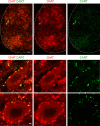

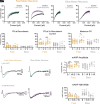
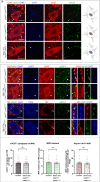
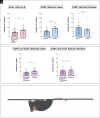
References
-
- Henneman E., Relation between size of neurons and their susceptibility to discharge. Science 126, 1345–1347 (1957). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

