Metal Binding of Alzheimer's Amyloid-β and Its Effect on Peptide Self-Assembly
- PMID: 37733746
- PMCID: PMC10552549
- DOI: 10.1021/acs.accounts.3c00370
Metal Binding of Alzheimer's Amyloid-β and Its Effect on Peptide Self-Assembly
Abstract
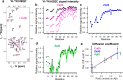
Metal ions have been identified as key factors modulating the aggregation of amyloid-β peptide (Aβ) implicated in Alzheimer's disease (AD). The presence of elevated levels of metal ions in the amyloid plaques in AD patients supports the notion that the dysfunction of metal homeostasis is connected to the development of AD pathology. Here, recent findings from high- and low-resolution biophysical techniques are put into perspective, providing detailed insights into the molecular structures and dynamics of metal-bound Aβ complexes and the effect of metal ions on the Aβ aggregation process. In particular, the development of theoretical kinetic models deducing different microscopic nucleation events from the macroscopic aggregation behavior has enabled deciphering of the effect of metal ions on specific nucleation processes. In addition to these macroscopic measurements of bulk aggregation to quantify microscopic rates, recent NMR studies have revealed details about the structures and dynamics of metal-Aβ complexes, thereby linking structural events to bulk aggregation. Interestingly, transition-metal ions, such as copper, zinc, and silver ions, form a compact complex with the N-terminal part of monomeric Aβ, respectively, where the metal-bound "folded" state is in dynamic equilibrium with an "unfolded" state. The rates and thermodynamic features of these exchange dynamics have been determined by using NMR relaxation dispersion experiments. Additionally, the application of specifically tailored paramagnetic NMR experiments on the Cu(II)-Aβ complex has been fruitful in obtaining structural constraints within the blind sphere of conventional NMR experiments. This enables the determination of molecular structures of the "folded" Cu(II)-coordinated N-terminal region of Aβ. Furthermore, the discussed transition-metal ions modulate Aβ self-assembly in a concentration-dependent manner, where low metal ion concentrations inhibit Aβ fibril formation, while at high metal ion concentrations other processes occur, resulting in amorphous aggregate formation. Remarkably, the metal-Aβ interaction predominately reduces one specific nucleation step, the fibril-end elongation, whereas primary and surface-catalyzed secondary nucleation mechanisms are less affected. Specific inhibition of fibril-end elongation theoretically predicts an enhanced generation of Aβ oligomers, which is an interesting contribution to understanding metal-Aβ-associated neurotoxic effects. Taken together, the metal binding process creates a metal-bound Aβ complex, which is seemingly inert to aggregation. This process hence efficiently reduces the aggregation-prone peptide pool, which on the macroscopic level is reflected as slower aggregation kinetics. Thus, the specific binding of metals to the Aβ monomer can be linked to the macroscopic inhibitory effect on Aβ bulk aggregation, providing a molecular understanding of the Aβ aggregation mechanism in the presence of metal ions, where the metal ion can be seen as a minimalist agent against Aβ self-assembly. These insights can help to target Aβ aggregation in vivo, where metal ions are key factors modulating the Aβ self-assembly and Aβ-associated neurotoxicity.
Conflict of interest statement
The author declares no competing financial interest.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

