PD-1 Blockade Induces Reactivation of Nonproductive T-Cell Responses Characterized by NF-κB Signaling in Patients with Pancreatic Cancer
- PMID: 37733830
- PMCID: PMC10831338
- DOI: 10.1158/1078-0432.CCR-23-1444
PD-1 Blockade Induces Reactivation of Nonproductive T-Cell Responses Characterized by NF-κB Signaling in Patients with Pancreatic Cancer
Abstract
Purpose: Pancreatic ductal adenocarcinoma (PDAC) trials have evaluated CTLA-4 and/or PD-(L)1 blockade in patients with advanced disease in which bulky tumor burden and limited time to develop antitumor T cells may have contributed to poor clinical efficacy. Here, we evaluated peripheral blood and tumor T cells from patients with PDAC receiving neoadjuvant chemoradiation plus anti-PD-1 (pembrolizumab) versus chemoradiation alone. We analyzed whether PD-1 blockade successfully reactivated T cells in the blood and/or tumor to determine whether lack of clinical benefit could be explained by lack of reactivated T cells versus other factors.
Experimental design: We used single-cell transcriptional profiling and TCR clonotype tracking to identify TCR clonotypes from blood that match clonotypes in the tumor.
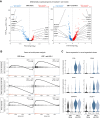
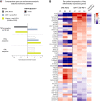
Results: PD-1 blockade increases the flux of TCR clonotypes entering cell cycle and induces an IFNγ signature like that seen in patients with other GI malignancies who respond to PD-1 blockade. However, these reactivated T cells have a robust signature of NF-κB signaling not seen in cases of PD-1 antibody response. Among paired samples between blood and tumor, several of the newly cycling clonotypes matched activated T-cell clonotypes observed in the tumor.
Conclusions: Cytotoxic T cells in the blood of patients with PDAC remain sensitive to reinvigoration by PD-1 blockade, and some have tumor-recognizing potential. Although these T cells proliferate and have a signature of IFN exposure, they also upregulate NF-κB signaling, which potentially counteracts the beneficial effects of anti-PD-1 reinvigoration and marks these T cells as non-productive contributors to antitumor immunity. See related commentary by Lander and DeNardo, p. 474.
©2023 The Authors; Published by the American Association for Cancer Research.
Figures



Comment in
-
Pancreatic Cancer's PD1-Roadblock: When T-Cell Reinvigoration Is Not Enough.Clin Cancer Res. 2024 Feb 1;30(3):474-476. doi: 10.1158/1078-0432.CCR-23-2991. Clin Cancer Res. 2024. PMID: 38038687 Free PMC article.
References
-
- Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res 2014;74:2913–21. - PubMed
-
- Kunzmann V, Siveke JT, Algul H, Goekkurt E, Siegler G, Martens U, et al. . Nab-paclitaxel plus gemcitabine versus nab-paclitaxel plus gemcitabine followed by FOLFIRINOX induction chemotherapy in locally advanced pancreatic cancer (NEOLAP-AIO-PAK-0113): a multicentre, randomised, phase 2 trial. Lancet Gastroenterol Hepatol 2021;6:128–38. - PubMed
-
- Hosein AN, Dougan SK, Aguirre AJ, Maitra A. Translational advances in pancreatic ductal adenocarcinoma therapy. Nat Cancer 2022;3:272–86. - PubMed
-
- Katz MHG, Shi Q, Meyers J, Herman JM, Chuong M, Wolpin BM, et al. . Efficacy of preoperative mFOLFIRINOX vs. mFOLFIRINOX plus hypofractionated radiotherapy for borderline resectable adenocarcinoma of the pancreas: the A021501 phase 2 randomized clinical trial. JAMA Oncol 2022;8:1263–70. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

