Biodistribution of mRNA COVID-19 vaccines in human breast milk
- PMID: 37734205
- PMCID: PMC10514401
- DOI: 10.1016/j.ebiom.2023.104800
Biodistribution of mRNA COVID-19 vaccines in human breast milk
Abstract
Background: COVID-19 mRNA vaccines play a vital role in the fight against SARS-CoV-2 infection. However, lactating women have been largely excluded from most vaccine clinical trials. As a result, limited research has been conducted on the systemic distribution of vaccine mRNA during lactation and whether it is excreted in human breast milk (BM). Here, we evaluated if COVID-19 vaccine mRNA is detectable in BM after maternal vaccination and determined its potential translational activity.
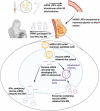
Methods: We collected BM samples from 13 lactating, healthy, post-partum women before and after COVID-19 mRNA vaccination. Vaccine mRNA in whole BM and BM extracellular vesicles (EVs) was assayed using quantitative Droplet Digital PCR, and its integrity and translational activity were evaluated.
Findings: Of 13 lactating women receiving the vaccine (20 exposures), trace mRNA amounts were detected in 10 exposures up to 45 h post-vaccination. The mRNA was concentrated in the BM EVs; however, these EVs neither expressed SARS-COV-2 spike protein nor induced its expression in the HT-29 cell line. Linkage analysis suggests vaccine mRNA integrity was reduced to 12-25% in BM.
Interpretation: Our findings demonstrate that the COVID-19 vaccine mRNA is not confined to the injection site but spreads systemically and is packaged into BM EVs. However, as only trace quantities are present and a clear translational activity is absent, we believe breastfeeding post-vaccination is safe, especially 48 h after vaccination. Nevertheless, since the minimum mRNA vaccine dose to elicit an immune reaction in infants <6 months is unknown, a dialogue between a breastfeeding mother and her healthcare provider should address the benefit/risk considerations of breastfeeding in the first two days after maternal vaccination.
Funding: This study was supported by the Department of Pediatrics, NYU-Grossman Long Island School of Medicine.
Keywords: Biodistribution; Breast milk; COVID-19; Extracellular vesicles; Lipid nanoparticles; Vaccine mRNA.
Copyright © 2023 The Author(s). Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of interests The authors have no conflict of interest.
Figures




References
-
- Dolgin E. The tangled history of mRNA vaccines. Nature. 2021;597(7876):318–324. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

