Novel insights into anticancer mechanisms of elesclomol: More than a prooxidant drug
- PMID: 37734229
- PMCID: PMC10518591
- DOI: 10.1016/j.redox.2023.102891
Novel insights into anticancer mechanisms of elesclomol: More than a prooxidant drug
Abstract
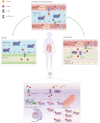
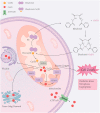
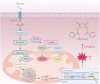
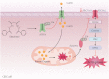
As an essential micronutrient for humans, the metabolism of copper is fine-tuned by evolutionarily conserved homeostatic mechanisms. Copper toxicity occurs when its concentration exceeds a certain threshold, which has been exploited in the development of copper ionophores, such as elesclomol, for anticancer treatment. Elesclomol has garnered recognition as a potent anticancer drug and has been evaluated in numerous clinical trials. However, the mechanisms underlying elesclomol-induced cell death remain obscure. The discovery of cuproptosis, a novel form of cell death triggered by the targeted accumulation of copper in mitochondria, redefines the significance of elesclomol in cancer therapy. Here, we provide an overview of copper homeostasis and its associated pathological disorders, especially copper metabolism in carcinogenesis. We summarize our current knowledge of the tumor suppressive mechanisms of elesclomol, with emphasis on cuproptosis. Finally, we discuss the strategies that may contribute to better application of elesclomol in cancer therapy.
Keywords: Cancer therapy; Copper metabolism; Cuproptosis; Elesclomol; Oxidative stress.
Copyright © 2023 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
Similar articles
-
Elesclomol: a copper ionophore targeting mitochondrial metabolism for cancer therapy.J Exp Clin Cancer Res. 2022 Sep 12;41(1):271. doi: 10.1186/s13046-022-02485-0. J Exp Clin Cancer Res. 2022. PMID: 36089608 Free PMC article. Review.
-
The oncology drug elesclomol selectively transports copper to the mitochondria to induce oxidative stress in cancer cells.Free Radic Biol Med. 2012 May 15;52(10):2142-50. doi: 10.1016/j.freeradbiomed.2012.03.017. Epub 2012 Apr 17. Free Radic Biol Med. 2012. PMID: 22542443
-
Copper Homeostasis Based on Cuproptosis-Related Signature Optimizes Molecular Subtyping and Treatment of Glioma.Mol Neurobiol. 2024 Aug;61(8):4962-4975. doi: 10.1007/s12035-023-03893-9. Epub 2023 Dec 28. Mol Neurobiol. 2024. PMID: 38151613
-
Elesclomol, a copper-transporting therapeutic agent targeting mitochondria: from discovery to its novel applications.J Transl Med. 2023 Oct 20;21(1):745. doi: 10.1186/s12967-023-04533-5. J Transl Med. 2023. PMID: 37864163 Free PMC article. Review.
-
Elesclomol induces copper-dependent ferroptosis in colorectal cancer cells via degradation of ATP7A.Mol Oncol. 2021 Dec;15(12):3527-3544. doi: 10.1002/1878-0261.13079. Epub 2021 Sep 15. Mol Oncol. 2021. PMID: 34390123 Free PMC article.
Cited by
-
An up-To-Date Review of Elesclomol and Its Nano-Formulations in Cancer Therapy.Cancer Rep (Hoboken). 2025 Apr;8(4):e70193. doi: 10.1002/cnr2.70193. Cancer Rep (Hoboken). 2025. PMID: 40195280 Free PMC article. Review.
-
A Copper-Based Photothermal-Responsive Nanoplatform Reprograms Tumor Immunogenicity via Self-Amplified Cuproptosis for Synergistic Cancer Therapy.Adv Sci (Weinh). 2025 May;12(19):e2500652. doi: 10.1002/advs.202500652. Epub 2025 Mar 24. Adv Sci (Weinh). 2025. PMID: 40125789 Free PMC article.
-
Targeting cuproptosis with nano material: new way to enhancing the efficacy of immunotherapy in colorectal cancer.Front Pharmacol. 2024 Dec 3;15:1451067. doi: 10.3389/fphar.2024.1451067. eCollection 2024. Front Pharmacol. 2024. PMID: 39691393 Free PMC article. Review.
-
Elesclomol Loaded Copper Oxide Nanoplatform Triggers Cuproptosis to Enhance Antitumor Immunotherapy.Adv Sci (Weinh). 2024 May;11(18):e2309984. doi: 10.1002/advs.202309984. Epub 2024 Mar 2. Adv Sci (Weinh). 2024. PMID: 38430531 Free PMC article.
-
Enzalutamide Sensitizes Castration-Resistant Prostate Cancer to Copper-Mediated Cell Death.Adv Sci (Weinh). 2024 Aug;11(30):e2401396. doi: 10.1002/advs.202401396. Epub 2024 Jun 10. Adv Sci (Weinh). 2024. PMID: 38859590 Free PMC article.
References
-
- Bleackley M.R., Macgillivray R.T. Transition metal homeostasis: from yeast to human disease. Biometals. 2011;24:785–809. - PubMed
-
- Denoyer D., Masaldan S., La Fontaine S., Cater M.A. Targeting copper in cancer therapy: 'Copper that Cancer'. Metallomics : Integrat. Biometal Sci. 2015;7:1459–1476. - PubMed
-
- Margalioth E.J., Schenker J.G., Chevion M. Copper and zinc levels in normal and malignant tissues. Cancer. 1983;52:868–872. - PubMed
-
- McAuslan B.R., Reilly W. Endothelial cell phagokinesis in response to specific metal ions. Exp. Cell Res. 1980;130:147–157. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

