Mpox infection protects against re-challenge in rhesus macaques
- PMID: 37734373
- PMCID: PMC10591870
- DOI: 10.1016/j.cell.2023.08.023
Mpox infection protects against re-challenge in rhesus macaques
Abstract
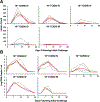
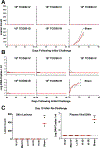
The mpox outbreak of 2022-2023 involved rapid global spread in men who have sex with men. We infected 18 rhesus macaques with mpox by the intravenous, intradermal, and intrarectal routes and observed robust antibody and T cell responses following all three routes of infection. Numerous skin lesions and high plasma viral loads were observed following intravenous and intradermal infection. Skin lesions peaked on day 10 and resolved by day 28 following infection. On day 28, we re-challenged all convalescent and 3 naive animals with mpox. All convalescent animals were protected against re-challenge. Transcriptomic studies showed upregulation of innate and inflammatory responses and downregulation of collagen formation and extracellular matrix organization following challenge, as well as rapid activation of T cell and plasma cell responses following re-challenge. These data suggest key mechanistic insights into mpox pathogenesis and immunity. This macaque model should prove useful for evaluating mpox vaccines and therapeutics.
Copyright © 2023 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures





References
-
- Grandpre LE, Duke-Cohan JS, Ewald BA, Devoy C, Barouch DH, Letvin NL, Reinherz EL, Baden LR, Dolin R, and Seaman MS (2009). Immunogenicity of recombinant Modified Vaccinia Ankara following a single or multi-dose vaccine regimen in rhesus monkeys. Vaccine 27, 1549–1556. S0264–410X(09)00020–6 [pii] 10.1016/j.vaccine.2009.01.010. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

