Neuroinflammation in the Dorsal Root Ganglia and Dorsal Horn Contributes to Persistence of Nociceptor Sensitization in SIV-Infected Antiretroviral Therapy-Treated Macaques
- PMID: 37734588
- PMCID: PMC10699130
- DOI: 10.1016/j.ajpath.2023.08.014
Neuroinflammation in the Dorsal Root Ganglia and Dorsal Horn Contributes to Persistence of Nociceptor Sensitization in SIV-Infected Antiretroviral Therapy-Treated Macaques
Abstract
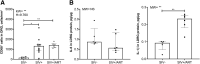
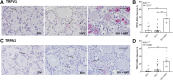
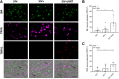
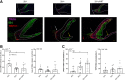
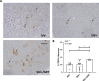
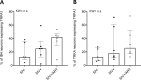
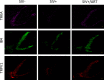
Despite the development of antiretroviral therapy (ART), HIV-associated distal sensory polyneuropathy remains prevalent. Using SIV-infected rhesus macaques, this study examined molecular mechanisms of peripheral and central sensitization to infer chronic pain from HIV infection. Previous studies identified atrophy in nociceptive neurons during SIV infection, which was associated with monocyte infiltration into the dorsal root ganglia (DRG). However, the sensory signaling mechanism connecting this pathology to symptoms remains unclear, especially because pain persists after resolution of high viremia and inflammation with ART. We hypothesized that residual DRG and dorsal horn neuroinflammation contributes to nociceptive sensitization. Using three cohorts of macaques [uninfected (SIV-), SIV-infected (SIV+), and SIV infected with ART (SIV+/ART)], this study showed an increase in the cellular and cytokine inflammatory profiles in the DRG of SIV+/ART macaques compared with uninfected animals. It found significant increase in the expression of nociceptive ion channels, TRPV1, and TRPA1 among DRG neurons in SIV+/ART compared with uninfected animals. SIV-infected and SIV+/ART animals showed reduced innervation of the nonpeptidergic nociceptors into the dorsal horn compared with uninfected animals. Finally, there were a significantly higher number of CD68+ cells in the dorsal horn of SIV+/ART macaques compared with uninfected animals. In summary, these data demonstrate that neuroinflammation, characteristics of nociceptor sensitization, and central terminal atrophy persists in SIV+/ART animals.
Copyright © 2023 American Society for Investigative Pathology. Published by Elsevier Inc. All rights reserved.
Figures




References
-
- Aziz-Donnelly A., Harrison T.B. Update of HIV-associated sensory neuropathies. Curr Treat Options Neurol. 2017;19:36. - PubMed
-
- Ellis R.J., Rosario D., Clifford D.B., McArthur J.C., Simpson D., Alexander T., Gelman B.B., Vaida F., Collier A., Marra C.M., Ances B., Atkinson J.H., Dworkin R.H., Morgello S., Grant I. Continued high prevalence and adverse clinical impact of human immunodeficiency virus–associated sensory neuropathy in the era of combination antiretroviral therapy. Arch Neurol. 2010;67:552–558. - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

