Thermodynamic forces from protein and water govern condensate formation of an intrinsically disordered protein domain
- PMID: 37735186
- PMCID: PMC10514047
- DOI: 10.1038/s41467-023-41586-y
Thermodynamic forces from protein and water govern condensate formation of an intrinsically disordered protein domain
Abstract
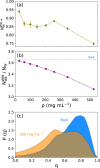
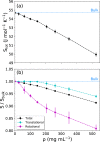
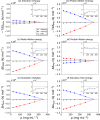
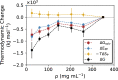
Liquid-liquid phase separation (LLPS) can drive a multitude of cellular processes by compartmentalizing biological cells via the formation of dense liquid biomolecular condensates, which can function as membraneless organelles. Despite its importance, the molecular-level understanding of the underlying thermodynamics of this process remains incomplete. In this study, we use atomistic molecular dynamics simulations of the low complexity domain (LCD) of human fused in sarcoma (FUS) protein to investigate the contributions of water and protein molecules to the free energy changes that govern LLPS. Both protein and water components are found to have comparably sizeable thermodynamic contributions to the formation of FUS condensates. Moreover, we quantify the counteracting effects of water molecules that are released into the bulk upon condensate formation and the waters retained within the protein droplets. Among the various factors considered, solvation entropy and protein interaction enthalpy are identified as the most important contributions, while solvation enthalpy and protein entropy changes are smaller. These results provide detailed molecular insights on the intricate thermodynamic interplay between protein- and solvation-related forces underlying the formation of biomolecular condensates.
© 2023. Springer Nature Limited.
Conflict of interest statement
The authors declare no competing interests.
Figures




Similar articles
-
Initial heat analysis in dissociation isothermal titration calorimetry: An analytical tool for thermodynamic dissection of biomolecular condensates.Biochem Biophys Res Commun. 2022 May 21;605:127-133. doi: 10.1016/j.bbrc.2022.03.063. Epub 2022 Mar 15. Biochem Biophys Res Commun. 2022. PMID: 35325654
-
Simulation of FUS Protein Condensates with an Adapted Coarse-Grained Model.J Chem Theory Comput. 2021 Jan 12;17(1):525-537. doi: 10.1021/acs.jctc.0c01064. Epub 2020 Dec 13. J Chem Theory Comput. 2021. PMID: 33307683 Free PMC article.
-
Heterogeneous Slowdown of Dynamics in the Condensate of an Intrinsically Disordered Protein.J Phys Chem Lett. 2024 Nov 14;15(45):11244-11251. doi: 10.1021/acs.jpclett.4c02142. Epub 2024 Nov 1. J Phys Chem Lett. 2024. PMID: 39486437 Free PMC article.
-
Liquid-liquid phase separation of intrinsically disordered proteins: Effect of osmolytes and crowders.Prog Mol Biol Transl Sci. 2025;211:249-269. doi: 10.1016/bs.pmbts.2024.11.005. Epub 2025 Jan 21. Prog Mol Biol Transl Sci. 2025. PMID: 39947751 Review.
-
Conformational Dynamics of Intrinsically Disordered Proteins Regulate Biomolecular Condensate Chemistry.Chem Rev. 2022 Mar 23;122(6):6719-6748. doi: 10.1021/acs.chemrev.1c00774. Epub 2022 Feb 18. Chem Rev. 2022. PMID: 35179885 Free PMC article. Review.
Cited by
-
Cytochrome c prompts the recruitment of its nuclear partners SET/TAF-Iβ and NPM1 into biomolecular condensates.iScience. 2024 Jul 2;27(8):110435. doi: 10.1016/j.isci.2024.110435. eCollection 2024 Aug 16. iScience. 2024. PMID: 39108706 Free PMC article.
-
Current practices in the study of biomolecular condensates: a community comment.Nat Commun. 2025 Aug 19;16(1):7730. doi: 10.1038/s41467-025-62055-8. Nat Commun. 2025. PMID: 40830340 Free PMC article.
-
Intracellular delivery strategies using membrane-interacting peptides and proteins.Nanoscale. 2024 Aug 22;16(33):15465-15480. doi: 10.1039/d4nr02093f. Nanoscale. 2024. PMID: 39091235 Free PMC article. Review.
-
Amino acid transfer free energies reveal thermodynamic driving forces in biomolecular condensate formation.Proc Natl Acad Sci U S A. 2025 Jul 29;122(30):e2425422122. doi: 10.1073/pnas.2425422122. Epub 2025 Jul 21. Proc Natl Acad Sci U S A. 2025. PMID: 40690668
-
Entropy Tug-of-War Determines Solvent Effects in the Liquid-Liquid Phase Separation of a Globular Protein.J Phys Chem Lett. 2024 Apr 18;15(15):4047-4055. doi: 10.1021/acs.jpclett.3c03421. Epub 2024 Apr 5. J Phys Chem Lett. 2024. PMID: 38580324 Free PMC article.
References
-
- Zimmerman, S. B. & Trach, S. O. Estimation of macromolecule concentrations and excluded volume effects for the cytoplasm of Escherichia coli. J. Mol. Biol.222, 599–620 (1991). - PubMed
-
- Wang, Y., Sarkar, M. Smith, A. E. Krois, A. S. & Pielak, G. J. Macromolecular crowding and protein stability. J. Am. Chem. Soc.134, 16614–16618 (2012). - PubMed
-
- Gao M, et al. Crowders and cosolvents-major contributors to the cellular milieu and efficient means to counteract environmental stresses. ChemPhysChem. 2017;18:2951–2972. - PubMed
-
- Barbosa, A. D., Savage, D. B. & Siniossoglou, S. Lipid droplet–organelle interactions: emerging roles in lipid metabolism. Curr. Opin. Cell Biol.35, 91–97 (2015). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

