The VCAM1-ApoE pathway directs microglial chemotaxis and alleviates Alzheimer's disease pathology
- PMID: 37735240
- PMCID: PMC10570140
- DOI: 10.1038/s43587-023-00491-1
The VCAM1-ApoE pathway directs microglial chemotaxis and alleviates Alzheimer's disease pathology
Abstract
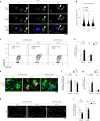
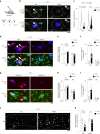
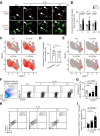
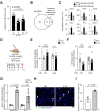
In Alzheimer's disease (AD), sensome receptor dysfunction impairs microglial danger-associated molecular pattern (DAMP) clearance and exacerbates disease pathology. Although extrinsic signals, including interleukin-33 (IL-33), can restore microglial DAMP clearance, it remains largely unclear how the sensome receptor is regulated and interacts with DAMP during phagocytic clearance. Here, we show that IL-33 induces VCAM1 in microglia, which promotes microglial chemotaxis toward amyloid-beta (Aβ) plaque-associated ApoE, and leads to Aβ clearance. We show that IL-33 stimulates a chemotactic state in microglia, characterized by Aβ-directed migration. Functional screening identified that VCAM1 directs microglial Aβ chemotaxis by sensing Aβ plaque-associated ApoE. Moreover, we found that disrupting VCAM1-ApoE interaction abolishes microglial Aβ chemotaxis, resulting in decreased microglial clearance of Aβ. In patients with AD, higher cerebrospinal fluid levels of soluble VCAM1 were correlated with impaired microglial Aβ chemotaxis. Together, our findings demonstrate that promoting VCAM1-ApoE-dependent microglial functions ameliorates AD pathology.
© 2023. The Author(s).
Conflict of interest statement
Y.J., A.K.F. and N.Y.I. are inventors on a patent licensed by The Hong Kong University of Science and Technology to Cognitact, and Y.J. co-founded Cognitact. D.M.H. is an inventor on a patent licensed by Washington University to C2N Diagnostics on the therapeutic use of anti-tau antibodies and on a patent licensed to NextCure on the therapeutic use of anti-ApoE antibodies. D.M.H. co-founded and is on the scientific advisory board of C2N Diagnostics. D.M.H. is also on the scientific advisory boards of Denali, Genentech and Cajal Neuroscience and consults for Alector. The remaining authors declare no competing interests.
Figures




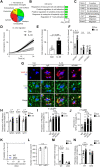
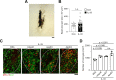
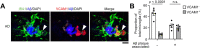

Comment in
-
ApoE attracts microglia to amyloid-β plaques.Nat Rev Neurol. 2023 Nov;19(11):639. doi: 10.1038/s41582-023-00885-0. Nat Rev Neurol. 2023. PMID: 37813951 No abstract available.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

