Hypoxic mesenchymal stem cell-derived exosomes promote the survival of skin flaps after ischaemia-reperfusion injury via mTOR/ULK1/FUNDC1 pathways
- PMID: 37735391
- PMCID: PMC10514998
- DOI: 10.1186/s12951-023-02098-5
Hypoxic mesenchymal stem cell-derived exosomes promote the survival of skin flaps after ischaemia-reperfusion injury via mTOR/ULK1/FUNDC1 pathways
Abstract
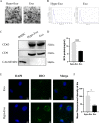
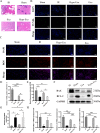
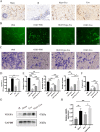
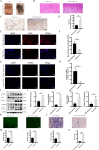
Flap necrosis, the most prevalent postoperative complication of reconstructive surgery, is significantly associated with ischaemia-reperfusion injury. Recent research indicates that exosomes derived from bone marrow mesenchymal stem cells (BMSCs) hold potential therapeutic applications in several diseases. Traditionally, BMSCs are cultured under normoxic conditions, a setting that diverges from their physiological hypoxic environment in vivo. Consequently, we propose a method involving the hypoxic preconditioning of BMSCs, aimed at exploring the function and the specific mechanisms of their exosomes in ischaemia-reperfusion skin flaps. This study constructed a 3 × 6 cm2 caudal superficial epigastric skin flap model and subjected it to ischaemic conditions for 6 h. Our findings reveal that exosomes from hypoxia-pretreated BMSCs significantly promoted flap survival, decrease MCP-1, IL-1β, and IL-6 levels in ischaemia-reperfusion injured flap, and reduce oxidative stress injury and apoptosis. Moreover, results indicated that Hypo-Exo provides protection to vascular endothelial cells from ischaemia-reperfusion injury both in vivo and in vitro. Through high-throughput sequencing and bioinformatics analysis, we further compared the differential miRNA expression profiles between Hypo-Exo and normoxic exosomes. Results display the enrichment of several pathways, including autophagy and mTOR. We have also elucidated a mechanism wherein Hypo-Exo promotes the survival of ischaemia-reperfusion injured flaps. This mechanism involves carrying large amounts of miR-421-3p, which target and regulate mTOR, thereby upregulating the expression of phosphorylated ULK1 and FUNDC1, and subsequently further activating autophagy. In summary, hypoxic preconditioning constitutes an effective and promising method for optimizing the therapeutic effects of BMSC-derived exosomes in the treatment of flap ischaemia-reperfusion injury.
Keywords: 3-MA; Autophagy; Bone marrow mesenchymal stem cells; Exosomes; Hypoxia; Ischaemia–reperfusion; Skin flap; miRNA.
© 2023. BioMed Central Ltd., part of Springer Nature.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures





References
-
- Varghese BT, Vijayakumar P, Ani Raj R, Thomas S. Salvaging skin loss of free fibular osteo-cutaneous flaps in oral oncological reconstruction. Oral Oncol. 2019;97:131–132. - PubMed
-
- Celik N, Wei F-C, Lin C-H, Cheng M-H, Chen H-C, Jeng S-F, Kuo Y-R. Technique and strategy in anterolateral thigh perforator flap surgery, based on an analysis of 15 complete and partial failures in 439 cases. Plast Reconstr Surg. 2002;109:2211–2216. - PubMed
-
- Geng L, Zhang G, Yao M, Fang Y. Rip 1-dependent endothelial necroptosis participates in ischemia-reperfusion injury of mouse flap. J Dermatol Sci. 2020;97:30–40. - PubMed
-
- Luo X, Zhao B, Chu T, Chen H, Li B, Li Z, Yan H. Improvement of multiterritory perforator flap survival supported by a hybrid perfusion mode: a novel strategy and literature review. J Tissue Viability. 2021;30:276–281. - PubMed
-
- Harder Y, Amon M, Laschke MW, et al. An old dream revitalised: preconditioning strategies to protect surgical flaps from critical ischaemia and ischaemia-reperfusion injury. J Plast Reconstr Aesthet Surg. 2008;61:503–511. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

