Nrf2 regulates iron-dependent hippocampal synapses and functional connectivity damage in depression
- PMID: 37735410
- PMCID: PMC10512501
- DOI: 10.1186/s12974-023-02875-x
Nrf2 regulates iron-dependent hippocampal synapses and functional connectivity damage in depression
Abstract
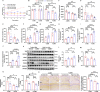
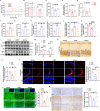
Neuronal iron overload contributes to synaptic damage and neuropsychiatric disorders. However, the molecular mechanisms underlying iron deposition in depression remain largely unexplored. Our study aims to investigate how nuclear factor-erythroid 2 (NF-E2)-related factor 2 (Nrf2) ameliorates hippocampal synaptic dysfunction and reduces brain functional connectivity (FC) associated with excessive iron in depression. We treated mice with chronic unpredictable mild stress (CUMS) with the iron chelator deferoxamine mesylate (DFOM) and a high-iron diet (2.5% carbonyl iron) to examine the role of iron overload in synaptic plasticity. The involvement of Nrf2 in iron metabolism and brain function was assessed using molecular biological techniques and in vivo resting-state functional magnetic resonance imaging (rs-fMRI) through genetic deletion or pharmacologic activation of Nrf2. The results demonstrated a significant correlation between elevated serum iron levels and impaired hippocampal functional connectivity (FC), which contributed to the development of depression-induced CUMS. Iron overload plays a crucial role in CUMS-induced depression and synaptic dysfunction, as evidenced by the therapeutic effects of a high-iron diet and DFOM. The observed iron overload in this study was associated with decreased Nrf2 levels and increased expression of transferrin receptors (TfR). Notably, inhibition of iron accumulation effectively attenuated CUMS-induced synaptic damage mediated by downregulation of brain-derived neurotrophic factor (BDNF). Nrf2-/- mice exhibited compromised FC within the limbic system and the basal ganglia, particularly in the hippocampus, and inhibition of iron accumulation effectively attenuated CUMS-induced synaptic damage mediated by downregulation of brain-derived neurotrophic factor (BDNF). Activation of Nrf2 restored iron homeostasis and reversed vulnerability to depression. Mechanistically, we further identified that Nrf2 deletion promoted iron overload via upregulation of TfR and downregulation of ferritin light chain (FtL), leading to BDNF-mediated synapse damage in the hippocampus. Therefore, our findings unveil a novel role for Nrf2 in regulating iron homeostasis while providing mechanistic insights into poststress susceptibility to depression. Targeting Nrf2-mediated iron metabolism may offer promising strategies for developing more effective antidepressant therapies.
Keywords: Depression; Iron metabolism; Nrf2; Rs-fMR; Synapse damage.
© 2023. BioMed Central Ltd., part of Springer Nature.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures





Similar articles
-
The enriched environment ameliorates chronic unpredictable mild stress-induced depressive-like behaviors and cognitive impairment by activating the SIRT1/miR-134 signaling pathway in hippocampus.J Affect Disord. 2019 Apr 1;248:81-90. doi: 10.1016/j.jad.2019.01.031. Epub 2019 Jan 28. J Affect Disord. 2019. PMID: 30716615
-
Antidepressant-like effect of ginsenoside Rb1 on potentiating synaptic plasticity via the miR-134-mediated BDNF signaling pathway in a mouse model of chronic stress-induced depression.J Ginseng Res. 2022 May;46(3):376-386. doi: 10.1016/j.jgr.2021.03.005. Epub 2021 Mar 20. J Ginseng Res. 2022. PMID: 35600767 Free PMC article.
-
Progressive alterations of hippocampal CA3-CA1 synapses in an animal model of depression.Behav Brain Res. 2014 Dec 15;275:191-200. doi: 10.1016/j.bbr.2014.08.040. Epub 2014 Sep 1. Behav Brain Res. 2014. PMID: 25192638
-
Remodeling of axo-spinous synapses in the pathophysiology and treatment of depression.Neuroscience. 2013 Oct 22;251:33-50. doi: 10.1016/j.neuroscience.2012.09.057. Epub 2012 Oct 2. Neuroscience. 2013. PMID: 23036622 Free PMC article. Review.
-
Nonalcoholic steatohepatitis and mechanisms by which it is ameliorated by activation of the CNC-bZIP transcription factor Nrf2.Free Radic Biol Med. 2022 Aug 1;188:221-261. doi: 10.1016/j.freeradbiomed.2022.06.226. Epub 2022 Jun 18. Free Radic Biol Med. 2022. PMID: 35728768 Review.
Cited by
-
Ferroptosis, pathogenesis and therapy in AS co-depression disease.Front Pharmacol. 2025 Feb 24;16:1516601. doi: 10.3389/fphar.2025.1516601. eCollection 2025. Front Pharmacol. 2025. PMID: 40066336 Free PMC article. Review.
-
Stress-Induced Cholesterol Metabolic Dysregulation and Differentiation Trajectory Shift in Oligodendrocytes Synergistically Drive Demyelination.Int J Mol Sci. 2025 Apr 9;26(8):3517. doi: 10.3390/ijms26083517. Int J Mol Sci. 2025. PMID: 40332029 Free PMC article.
-
The Antinociceptive Role of Nrf2 in Neuropathic Pain: From Mechanisms to Clinical Perspectives.Pharmaceutics. 2024 Aug 15;16(8):1068. doi: 10.3390/pharmaceutics16081068. Pharmaceutics. 2024. PMID: 39204413 Free PMC article. Review.
-
Ferroptosis-associated signaling pathways and therapeutic approaches in depression.Front Neurosci. 2025 Mar 19;19:1559597. doi: 10.3389/fnins.2025.1559597. eCollection 2025. Front Neurosci. 2025. PMID: 40177374 Free PMC article. Review.
-
Exploring the Effect and Mechanism of Liraglutide in Treating Depression Based on Network Pharmacology and Experimental Analysis.J Cell Mol Med. 2025 Jun;29(11):e70630. doi: 10.1111/jcmm.70630. J Cell Mol Med. 2025. PMID: 40461955 Free PMC article.
References
-
- Malhi GS, Mann JJ. Depression. Lancet. 2018;392:2299–2312. - PubMed
MeSH terms
Substances
Grants and funding
- 2022RC01/Young Science and Technology Talents Project of The Affiliated TCM Hospital of Guangzhou Medical University
- No. 20201089/Administration of Traditional Chinese Medicine of Guangdong Province
- 81230085/National Natural Science Foundation of China
- 81873170/National Natural Science Foundation of China
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

