Q fever and toxoplasmosis in South African livestock and wildlife: a retrospective study on seropositivity, sporadic abortion, and stillbirth cases in livestock caused by Coxiella burnetii
- PMID: 37735412
- PMCID: PMC10512517
- DOI: 10.1186/s12917-023-03645-w
Q fever and toxoplasmosis in South African livestock and wildlife: a retrospective study on seropositivity, sporadic abortion, and stillbirth cases in livestock caused by Coxiella burnetii
Abstract
Background: Q fever and toxoplasmosis are economically important zoonoses as they cause considerable losses in livestock (cattle, sheep and goats) and wildlife (antelopes, giraffes, lions, and cheetahs) through reproductive disorders such as abortions and stillbirths. Q fever and toxoplasmosis testing in South Africa is conducted by the Agricultural Research Council-Onderstepoort Veterinary Research (ARC-OVR). However, both zoonoses are understudied and not monitored in South Africa as they are not considered controlled or notifiable diseases in the Animal Disease Act 35 of 1984. A retrospective study was conducted on Q fever (2007-2009) and toxoplasmosis (2007-2017) using diagnostic laboratory data at the ARC-OVR. Also, we report on sporadic abortion and stillbirth cases in livestock from diagnostic tissue samples submitted for Coxiella burnetii polymerase chain reaction (PCR) detection at the ARC-OVR.
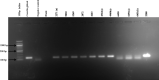
Results: During 2007 to 2009, 766 animal samples were tested for C. burnetii antibodies and seropositivity was 0.9% (95%CI: 0.3-1.7) with sheep (1.9%; 95%CI: 0.6-4.4) having the highest seropositivity followed by cattle (0.7%; 95%CI: 0.09-2.6), while all goats (0.0%; 95%CI: 0.0-4.2) and wildlife (0.0%; 95%CI: 0.0-2.5) tested were negative. From 2007 to 2017, 567 sera were tested for T. gondii antibodies; overall seropositivity was 12.2% (95%CI: 9.6-15). Wildlife had highest seropositivity to T. gondii antibodies (13.9%; 95%CI: 9.0-19.7) followed by goats (12.9%; 95%CI: 9.2-17.4) and sheep (12.3%; 95%CI: 5.1-23.8) while seropositivity in cattle was 2.4% (95%CI: 0.06-12.9). Of 11 animals tested by C. burnetii PCR detection (2021-2022), 10 (91.0%) were positive. The amplicon sequences showed similarity to Coxiella burnetii strain 54T1 transposase gene partial coding sequence.
Conclusions: We have confirmed the occurrence of the causative agents of Q fever and toxoplasmosis in livestock and wildlife in South Africa, with data limitations. These zoonoses remain of importance with limited information about them in South Africa. This study provides baseline information for future studies on Q fever and toxoplasmosis in South African livestock and wildlife, as well other African countries. Due to limited data collection experienced in this study, it is recommended that improvements in data collection samples tested should include associated factors such as sex, age, and breed of the animals.
Keywords: Diagnostic laboratory data; PCR detection; Retrospective study; Risk factors; Seropositivity.
© 2023. BioMed Central Ltd., part of Springer Nature.
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Molecular detection of Coxiella burnetii infection in aborted samples of domestic ruminants in Iran.PLoS One. 2021 Apr 14;16(4):e0250116. doi: 10.1371/journal.pone.0250116. eCollection 2021. PLoS One. 2021. PMID: 33852632 Free PMC article.
-
Epidemiology of brucellosis, Q Fever and Rift Valley Fever at the human and livestock interface in northern Côte d'Ivoire.Acta Trop. 2017 Jan;165:66-75. doi: 10.1016/j.actatropica.2016.02.012. Epub 2016 Feb 17. Acta Trop. 2017. PMID: 26899680
-
Coxiella burnetii DNA detected in domestic ruminants and wildlife from Portugal.Vet Microbiol. 2015 Oct 22;180(1-2):136-41. doi: 10.1016/j.vetmic.2015.07.030. Epub 2015 Jul 28. Vet Microbiol. 2015. PMID: 26345258
-
The relevance of the wild reservoir in zoonotic multi-host pathogens: The links between Iberian wild mammals and Coxiella burnetii.Transbound Emerg Dis. 2022 Nov;69(6):3868-3880. doi: 10.1111/tbed.14758. Epub 2022 Nov 26. Transbound Emerg Dis. 2022. PMID: 36335588 Review.
-
Coxiella burnetii infections in sheep or goats: an opinionated review.Vet Microbiol. 2015 Dec 14;181(1-2):119-29. doi: 10.1016/j.vetmic.2015.07.011. Epub 2015 Jul 15. Vet Microbiol. 2015. PMID: 26315774 Review.
Cited by
-
The Microbiome and Coxiella Diversity Found in Amblyomma hebraeum and Dermacentor rhinocerinus Ticks Sampled from White Rhinoceros.Microb Ecol. 2025 May 22;88(1):48. doi: 10.1007/s00248-025-02549-6. Microb Ecol. 2025. PMID: 40402315 Free PMC article.
-
Reproduction and Productivity in Dairy Cattle after Abortions Both Related and Unrelated to Coxiella burnetii.Animals (Basel). 2023 Nov 18;13(22):3561. doi: 10.3390/ani13223561. Animals (Basel). 2023. PMID: 38003178 Free PMC article.
-
Coxiella burnetii (Q Fever) in Small Ruminants on Farms in North West Province, South Africa.Vet Sci. 2025 Mar 31;12(4):315. doi: 10.3390/vetsci12040315. Vet Sci. 2025. PMID: 40284817 Free PMC article.
-
Serological Evidence and Coexposure of Selected Infections among Livestock Slaughtered at Eastern Cape Abattoirs in South Africa.Int J Microbiol. 2023 Dec 1;2023:8906971. doi: 10.1155/2023/8906971. eCollection 2023. Int J Microbiol. 2023. PMID: 38077996 Free PMC article.
-
Coxiella burnetii and Reproductive Disorders in Cattle: A Systematic Review.Animals (Basel). 2024 Apr 27;14(9):1313. doi: 10.3390/ani14091313. Animals (Basel). 2024. PMID: 38731318 Free PMC article. Review.
References
-
- Kittelberger R, Mars J, Wibberley G, Sting R, Henning K, Horner GW, et al. Comparison of the q-fever complement fixation test and two commercial enzyme-linked immunosorbent assays for the detection of serum antibodies against Coxiella burnetii (Q-fever) in ruminants: Recommendations for use of serological tests on imported animals in New Zealand. N Z Vet J. 2009;57(5):262–268. doi: 10.1080/00480169.2009.58619. - DOI - PubMed
-
- Angelakis E, Raoult D. Q fever. Vet Microbiol. 2010;40:(3-4):297–309. - PubMed
-
- Arricau-Bouvery N, Rodolakis A. Is Q fever an emerging or re-emerging zoonosis? Vet Res. 2005;36(3):327–49. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

