Retinal ganglion cell repopulation for vision restoration in optic neuropathy: a roadmap from the RReSTORe Consortium
- PMID: 37735444
- PMCID: PMC10514988
- DOI: 10.1186/s13024-023-00655-y
Retinal ganglion cell repopulation for vision restoration in optic neuropathy: a roadmap from the RReSTORe Consortium
Abstract
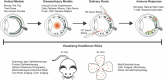
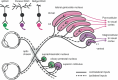
Retinal ganglion cell (RGC) death in glaucoma and other optic neuropathies results in irreversible vision loss due to the mammalian central nervous system's limited regenerative capacity. RGC repopulation is a promising therapeutic approach to reverse vision loss from optic neuropathies if the newly introduced neurons can reestablish functional retinal and thalamic circuits. In theory, RGCs might be repopulated through the transplantation of stem cell-derived neurons or via the induction of endogenous transdifferentiation. The RGC Repopulation, Stem Cell Transplantation, and Optic Nerve Regeneration (RReSTORe) Consortium was established to address the challenges associated with the therapeutic repair of the visual pathway in optic neuropathy. In 2022, the RReSTORe Consortium initiated ongoing international collaborative discussions to advance the RGC repopulation field and has identified five critical areas of focus: (1) RGC development and differentiation, (2) Transplantation methods and models, (3) RGC survival, maturation, and host interactions, (4) Inner retinal wiring, and (5) Eye-to-brain connectivity. Here, we discuss the most pertinent questions and challenges that exist on the path to clinical translation and suggest experimental directions to propel this work going forward. Using these five subtopic discussion groups (SDGs) as a framework, we suggest multidisciplinary approaches to restore the diseased visual pathway by leveraging groundbreaking insights from developmental neuroscience, stem cell biology, molecular biology, optical imaging, animal models of optic neuropathy, immunology & immunotolerance, neuropathology & neuroprotection, materials science & biomedical engineering, and regenerative neuroscience. While significant hurdles remain, the RReSTORe Consortium's efforts provide a comprehensive roadmap for advancing the RGC repopulation field and hold potential for transformative progress in restoring vision in patients suffering from optic neuropathies.
Keywords: Glaucoma; Neuroprotection; Ophthalmology; Optic neuropathy; Organoids; Regenerative medicine; Retinal ganglion cells; Stem cells; Transplantation.
© 2023. Editorial Group and BioMed Central Ltd., part of Springer Nature.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
-
- Goldberg JL, Espinosa JS, Xu Y, Davidson N, Kovacs GTA, Barres BA. Retinal ganglion cells do not extend axons by default promotion by neurotrophic signaling and electrical activity. Neuron. 2002;33:689–702. - PubMed
Publication types
MeSH terms
Grants and funding
- R01 EY015290/EY/NEI NIH HHS/United States
- R01 EY028148/EY/NEI NIH HHS/United States
- R13 EY034018/EY/NEI NIH HHS/United States
- R00 EY029373/EY/NEI NIH HHS/United States
- R01 EY031817/EY/NEI NIH HHS/United States
- F32 EY033211/EY/NEI NIH HHS/United States
- K08 EY033032/EY/NEI NIH HHS/United States
- R01 EY029739/EY/NEI NIH HHS/United States
- R01 EY027920/EY/NEI NIH HHS/United States
- K08 EY031797/EY/NEI NIH HHS/United States
- K08 EY031801/EY/NEI NIH HHS/United States
- P30 EY014800/EY/NEI NIH HHS/United States
- R21 EY034332/EY/NEI NIH HHS/United States

