Decoding the endometrial niche of Asherman's Syndrome at single-cell resolution
- PMID: 37735465
- PMCID: PMC10514053
- DOI: 10.1038/s41467-023-41656-1
Decoding the endometrial niche of Asherman's Syndrome at single-cell resolution
Abstract
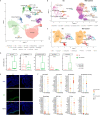
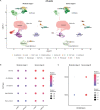
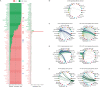
Asherman's Syndrome is characterized by intrauterine adhesions or scarring, which cause infertility, menstrual abnormalities, and recurrent pregnancy loss. The pathophysiology of this syndrome remains unknown, with treatment restricted to recurrent surgical removal of intrauterine scarring, which has limited success. Here, we decode the Asherman's Syndrome endometrial cell niche by analyzing data from over 200,000 cells with single-cell RNA-sequencing in patients with this condition and through in vitro analyses of Asherman's Syndrome patient-derived endometrial organoids. Our endometrial atlas highlights the loss of the endometrial epithelium, alterations to epithelial differentiation signaling pathways such as Wnt and Notch, and the appearance of characteristic epithelium expressing secretory leukocyte protease inhibitor during the window of implantation. We describe syndrome-associated alterations in cell-to-cell communication and gene expression profiles that support a dysfunctional pro-fibrotic, pro-inflammatory, and anti-angiogenic environment.
© 2023. Springer Nature Limited.
Conflict of interest statement
C.S. and X.S. are founders and shareholders of Asherman Therapy S.L. and inventors of patents granted in Europe, Australia, Canada, Japan, Russian Federation, Mexico, and the US derived from PCT application PCT/IB2015/001715. M.P-F. is partially employed by Asherman Therapy S.L. The remaining authors declare no competing interests.
Figures



References
-
- Dmowski WP, Greenblatt RB. Asherman’s syndrome and risk of placenta accreta. Obstet. Gynecol. 1969;34:288–299. - PubMed
-
- Public summary of opinion on orphan designation. Committee Orphan Medicinal Products (COMP). EMA/206895/2017 (2017).
-
- Wallach EE, Schenker JG, Margalioth EJ. Intrauterine adhesions: an updated appraisal. Fertil. Steril. 1982;37:593–610. - PubMed
-
- Yu D, Wong Y-M, Cheong Y, Xia E, Li T-C. Asherman syndrome—one century later. Fertil. Steril. 2008;89:759–779. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

