Targeting ferroptosis opens new avenues for the development of novel therapeutics
- PMID: 37735472
- PMCID: PMC10514338
- DOI: 10.1038/s41392-023-01606-1
Targeting ferroptosis opens new avenues for the development of novel therapeutics
Abstract
Ferroptosis is an iron-dependent form of regulated cell death with distinct characteristics, including altered iron homeostasis, reduced defense against oxidative stress, and abnormal lipid peroxidation. Recent studies have provided compelling evidence supporting the notion that ferroptosis plays a key pathogenic role in many diseases such as various cancer types, neurodegenerative disease, diseases involving tissue and/or organ injury, and inflammatory and infectious diseases. Although the precise regulatory networks that underlie ferroptosis are largely unknown, particularly with respect to the initiation and progression of various diseases, ferroptosis is recognized as a bona fide target for the further development of treatment and prevention strategies. Over the past decade, considerable progress has been made in developing pharmacological agonists and antagonists for the treatment of these ferroptosis-related conditions. Here, we provide a detailed overview of our current knowledge regarding ferroptosis, its pathological roles, and its regulation during disease progression. Focusing on the use of chemical tools that target ferroptosis in preclinical studies, we also summarize recent advances in targeting ferroptosis across the growing spectrum of ferroptosis-associated pathogenic conditions. Finally, we discuss new challenges and opportunities for targeting ferroptosis as a potential strategy for treating ferroptosis-related diseases.
© 2023. West China Hospital, Sichuan University.
Conflict of interest statement
The authors declare no competing interests.
Figures



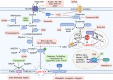


References
-
- Friedmann Angeli JP, Krysko DV, Conrad M. Ferroptosis at the crossroads of cancer-acquired drug resistance and immune evasion. Nat. Rev. Cancer. 2019;19:405–414. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

