Somatostatin neurons in prefrontal cortex initiate sleep-preparatory behavior and sleep via the preoptic and lateral hypothalamus
- PMID: 37735497
- PMCID: PMC10545541
- DOI: 10.1038/s41593-023-01430-4
Somatostatin neurons in prefrontal cortex initiate sleep-preparatory behavior and sleep via the preoptic and lateral hypothalamus
Erratum in
-
Author Correction: Somatostatin neurons in prefrontal cortex initiate sleep-preparatory behavior and sleep via the preoptic and lateral hypothalamus.Nat Neurosci. 2025 Jul;28(7):1570. doi: 10.1038/s41593-025-02003-3. Nat Neurosci. 2025. PMID: 40473854 Free PMC article. No abstract available.
Abstract
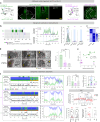
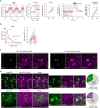
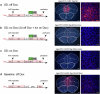
The prefrontal cortex (PFC) enables mammals to respond to situations, including internal states, with appropriate actions. One such internal state could be 'tiredness'. Here, using activity tagging in the mouse PFC, we identified particularly excitable, fast-spiking, somatostatin-expressing, γ-aminobutyric acid (GABA) (PFCSst-GABA) cells that responded to sleep deprivation. These cells projected to the lateral preoptic (LPO) hypothalamus and the lateral hypothalamus (LH). Stimulating PFCSst-GABA terminals in the LPO hypothalamus caused sleep-preparatory behavior (nesting, elevated theta power and elevated temperature), and stimulating PFCSst-GABA terminals in the LH mimicked recovery sleep (non-rapid eye-movement sleep with higher delta power and lower body temperature). PFCSst-GABA terminals had enhanced activity during nesting and sleep, inducing inhibitory postsynaptic currents on diverse cells in the LPO hypothalamus and the LH. The PFC also might feature in deciding sleep location in the absence of excessive fatigue. These findings suggest that the PFC instructs the hypothalamus to ensure that optimal sleep takes place in a suitable place.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
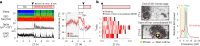














References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

