Strong associations of telomere length and mitochondrial copy number with suicidality and abuse history in adolescent depressed individuals
- PMID: 37735501
- PMCID: PMC10730407
- DOI: 10.1038/s41380-023-02263-0
Strong associations of telomere length and mitochondrial copy number with suicidality and abuse history in adolescent depressed individuals
Abstract
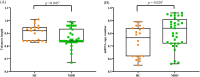
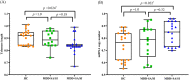
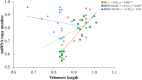
Major depressive disorder (MDD) is highly prevalent in adolescents and is a major risk factor for suicidality. Recent evidence shows that accelerated cellular senescence/aging is associated with psychiatric illness, including depression, in adults. The present study examined if the relationships of telomere length (TL) and mitochondrial DNA copy number (mtDNAcn), two critical indicators of cellular senescence/aging, are altered in depressed adolescents and whether these alterations are associated with suicidality, early-life adversities, and other co-occuring factors. In genomic DNA isolated from 53 adolescents (ages 16-19, 19 MDD with suicide attempt/suicidal ideation [MDD + SI/SA], 14 MDD without SA/SI [MDD-SI/SA], and 20 healthy controls [HC]), TL and mtDNAcn were measured as the ratio between the number of telomere repeats and that of a single-copy nuclear-hemoglobin [HBG] gene or the amount of mtDNA (NADH dehydrogenase, subunit 1) relative to HBG. Our data show that TL was significantly lower, and mtDNAcn was significantly higher in the total MDD group than HC. TL was significantly lower and mtDNAcn was significantly higher in the MDD + SA/SI group than in the HC, whereas there were no differences in the MDD-SI/SA group. TL was positively correlated with mtDNAcn in both HC and MDD-SA/SI groups; however, TL was negatively correlated with mtDNAcn in MDD + SA/SI. Furthermore, TL was negatively correlated with the severity of both depression and anxiety, while mtDNAcn was positively correlated with the severity of prior emotional abuse. Our study indicates that cellular senescence is more advanced in depressed adolescents with suicidal ideation and that childhood emotional abuse may participate in such a process.
© 2023. The Author(s).
Conflict of interest statement
RCS has received grant support from the National Institutes of Health, Patient-Centered Outcomes Research Institute, American Foundation for Suicide Prevention, Acadia Pharmaceuticals, Alkermes, Inc., Allergan, Plc, Alto Pharmaceuticals, Avanir Pharmaceuticals, Inc., Boehringer Ingelheim, Denovo Biopharma, Genomind, InMune Bio, Intra-cellular Therapies, Janssen Pharmaceuticals, LivaNova PLC, Navitor Pharmaceuticals, Neurocrine Biosciences, Neurorx, Novartis Pharmaceuticals, Otsuka Pharmaceutical Company, Sunovion Pharmaceuticals, and Takeda Pharmaceuticals. He has received consulting fees from Acadia Pharmaceuticals, Allergan, Plc, Alphasigma USA, Inc., Boehringer Ingelheim, Cerecor, Inc., Denovo Biopharma, Janssen Pharmaceuticals, Myriad Neuroscience, Neurorx, Novartis AG, Otsuka Pharmaceuticals, Seelos Therapeutics, Inc., Sunovion Pharmaceuticals, and Takeda Pharmaceuticals. Other authors report no conflict of interest.
Figures

References
-
- Emslie GJ, Ventura D, Korotzer A, Tourkodimitris S. Escitalopram in the treatment of adolescent depression: a randomized placebo-controlled multisite trial. J Am Acad Child Adolesc Psychiatry. 2009;48:721–9. - PubMed
-
- Daly M. Prevalence of depression among adolescents in the U.S. from 2009 to 2019: analysis of trends by sex, race/ethnicity, and income. J Adolesc Health. 2022;70:496–9. - PubMed
-
- Grossberg A, Rice T. Depression and suicidal behavior in adolescents. Med Clin North Am. 2022;107:169–82. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

