Structure guided mimicry of an essential P. falciparum receptor-ligand complex enhances cross neutralizing antibodies
- PMID: 37735574
- PMCID: PMC10514071
- DOI: 10.1038/s41467-023-41636-5
Structure guided mimicry of an essential P. falciparum receptor-ligand complex enhances cross neutralizing antibodies
Abstract
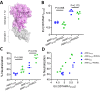
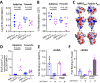
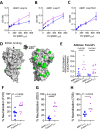
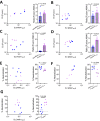
Invasion of human erythrocytes by Plasmodium falciparum (Pf) merozoites relies on the interaction between two parasite proteins: apical membrane antigen 1 (AMA1) and rhoptry neck protein 2 (RON2). While antibodies to AMA1 provide limited protection against Pf in non-human primate malaria models, clinical trials using recombinant AMA1 alone (apoAMA1) yielded no protection due to insufficient functional antibodies. Immunization with AMA1 bound to RON2L, a 49-amino acid peptide from its ligand RON2, has shown superior protection by increasing the proportion of neutralizing antibodies. However, this approach relies on the formation of a complex in solution between the two vaccine components. To advance vaccine development, here we engineered chimeric antigens by replacing the AMA1 DII loop, displaced upon ligand binding, with RON2L. Structural analysis confirmed that the fusion chimera (Fusion-FD12) closely mimics the binary AMA1-RON2L complex. Immunization studies in female rats demonstrated that Fusion-FD12 immune sera, but not purified IgG, neutralized vaccine-type parasites more efficiently compared to apoAMA1, despite lower overall anti-AMA1 titers. Interestingly, Fusion-FD12 immunization enhanced antibodies targeting conserved epitopes on AMA1, leading to increased neutralization of non-vaccine type parasites. Identifying these cross-neutralizing antibody epitopes holds promise for developing an effective, strain-transcending malaria vaccine.
© 2023. Springer Nature Limited.
Conflict of interest statement
P.S. is a named inventor on patents related to this work. The remaining authors declare no competing interests.
Figures

Update of
-
Structure guided mimicry of an essential P. falciparum receptor-ligand complex enhances cross neutralizing antibodies.Res Sq [Preprint]. 2023 Apr 20:rs.3.rs-2733434. doi: 10.21203/rs.3.rs-2733434/v1. Res Sq. 2023. Update in: Nat Commun. 2023 Sep 21;14(1):5879. doi: 10.1038/s41467-023-41636-5. PMID: 37131813 Free PMC article. Updated. Preprint.
References
-
- WHO. World Malaria Report. (World Health Organization, 2021).
-
- Langhorne J, Ndungu FM, Sponaas AM, Marsh K. Immunity to malaria: more questions than answers. Nat. Immunol. 2008;9:725–732. - PubMed
-
- Triglia T, et al. Apical membrane antigen 1 plays a central role in erythrocyte invasion by Plasmodium species. Mol. Microbiol. 2000;38:706–718. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

