A Versatile PDA(DOX) Nanoplatform for Chemo-Photothermal Synergistic Therapy against Breast Cancer and Attenuated Doxorubicin-Induced Cardiotoxicity
- PMID: 37735669
- PMCID: PMC10512561
- DOI: 10.1186/s12951-023-02072-1
A Versatile PDA(DOX) Nanoplatform for Chemo-Photothermal Synergistic Therapy against Breast Cancer and Attenuated Doxorubicin-Induced Cardiotoxicity
Abstract
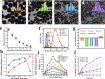
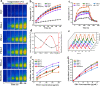
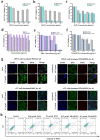
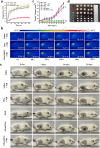
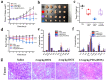
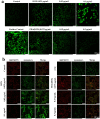
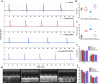
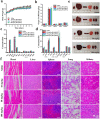
Photothermal therapy (PTT) is a highly clinical application promising cancer treatment strategy with safe, convenient surgical procedures and excellent therapeutic efficacy on superficial tumors. However, a single PTT is difficult to eliminate tumor cells completely, and tumor recurrence and metastasis are prone to occur in the later stage. Chemo-photothermal synergistic therapy can conquer the shortcomings by further killing residual tumor cells after PTT through systemic chemotherapy. Nevertheless, chemotherapy drugs' extreme toxicity is also a problematic issue to be solved, such as anthracycline-induced cardiotoxicity. Herein, we selected polydopamine nanoparticles (PDA) as the carrier of the chemotherapeutic drug doxorubicin (DOX) to construct a versatile PDA(DOX) nanoplatform for chemo-photothermal synergistic therapy against breast cancer and simultaneously attenuated DOX-induced cardiotoxicity (DIC). The excellent photothermal properties of PDA were used to achieve the thermal ablation of tumors. DOX carried out chemotherapy to kill residual and occult distant tumors. Furthermore, the PDA(DOX) nanoparticles significantly alleviate DIC, which benefits from PDA's excellent antioxidant enzyme activity. The experimental data of the chemotherapy groups showed that the results of the PDA(DOX) group were much better than the DOX group. This study not only effectively inhibits cancer but tactfully attenuates DIC, bringing a new perspective into synergistic therapy against breast cancer.
Keywords: Doxorubicin-induced cardiotoxicity; Oxidative stress; Photothermal-chemotherapy synergistic therapy; Polydopamine.
© 2023. BioMed Central Ltd., part of Springer Nature.
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
Targeted polydopamine nanoparticles enable photoacoustic imaging guided chemo-photothermal synergistic therapy of tumor.Acta Biomater. 2017 Jan 1;47:124-134. doi: 10.1016/j.actbio.2016.10.010. Epub 2016 Oct 6. Acta Biomater. 2017. PMID: 27721008
-
Construction of Surface-Modified Polydopamine Nanoparticles for Sequential Drug Release and Combined Chemo-Photothermal Cancer Therapy.Mol Pharm. 2021 Mar 1;18(3):1327-1343. doi: 10.1021/acs.molpharmaceut.0c01164. Epub 2021 Feb 2. Mol Pharm. 2021. PMID: 33530691
-
Fabricating polydopamine-coated MoSe2-wrapped hollow mesoporous silica nanoplatform for controlled drug release and chemo-photothermal therapy.Int J Nanomedicine. 2018 Nov 16;13:7607-7621. doi: 10.2147/IJN.S181681. eCollection 2018. Int J Nanomedicine. 2018. PMID: 30510420 Free PMC article.
-
Potential of exosomes as diagnostic biomarkers and therapeutic carriers for doxorubicin-induced cardiotoxicity.Int J Biol Sci. 2021 Mar 27;17(5):1328-1338. doi: 10.7150/ijbs.58786. eCollection 2021. Int J Biol Sci. 2021. PMID: 33867849 Free PMC article. Review.
-
Anthracycline-induced cardiotoxicity: targeting high-density lipoproteins to limit the damage?Lipids Health Dis. 2022 Sep 1;21(1):85. doi: 10.1186/s12944-022-01694-y. Lipids Health Dis. 2022. PMID: 36050733 Free PMC article. Review.
Cited by
-
N-doped carbon dots for dual-modality NIR fluorescence imaging and photothermal therapy.J Nanobiotechnology. 2025 Jul 15;23(1):513. doi: 10.1186/s12951-025-03497-6. J Nanobiotechnology. 2025. PMID: 40660215 Free PMC article.
-
Natural epigallocatechin-3-gallocarboxylate nanoformulation loaded doxorubicin to construct a novel and low cardiotoxicity chemotherapeutic drug for high-efficiency breast cancer therapy.J Nanobiotechnology. 2024 Dec 24;22(1):793. doi: 10.1186/s12951-024-03069-0. J Nanobiotechnology. 2024. PMID: 39719646 Free PMC article.
-
Pleiotropic Multi-Drug Co-Assembled Nanocomposites Offer Protection Against Doxorubicin-Induced Cardiotoxicity.Int J Nanomedicine. 2025 Jul 23;20:9311-9326. doi: 10.2147/IJN.S528349. eCollection 2025. Int J Nanomedicine. 2025. PMID: 40727580 Free PMC article.
References
-
- Gomez-Cambronero J. Lack of effective translational regulation of PLD expression and exosome biogenesis in triple-negative breast cancer cells. Cancer Metastasis Rev. 2018;37(2–3):491–507. - PubMed
-
- Bonferoni MC, Rossi S, Sandri G, Ferrari F. Nanoparticle formulations to enhance tumor targeting of poorly soluble polyphenols with potential anticancer properties. Semin Cancer Biol. 2017;46:205–214. - PubMed
MeSH terms
Substances
Grants and funding
- 2022R432A026/College Students' Science and Technology Innovation Project of Zhejiang Province
- YB202219/Zhejiang University Laboratory Work Research Project
- 2021KY1159/Medical and Health Science and Technology Plan Project of Zhejiang Province
- 2020A13024/Medical and Health Science and Technology Plan Project of Shaoxing
- 2020A13060/Medical and Health Science and Technology Plan Project of Shaoxing
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

