The role of bone marrow microenvironment (BMM) cells in acute myeloid leukemia (AML) progression: immune checkpoints, metabolic checkpoints, and signaling pathways
- PMID: 37735675
- PMCID: PMC10512514
- DOI: 10.1186/s12964-023-01282-2
The role of bone marrow microenvironment (BMM) cells in acute myeloid leukemia (AML) progression: immune checkpoints, metabolic checkpoints, and signaling pathways
Abstract
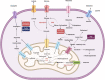
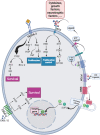
Acute myeloid leukemia (AML) comprises a multifarious and heterogeneous array of illnesses characterized by the anomalous proliferation of myeloid cells in the bone marrow microenvironment (BMM). The BMM plays a pivotal role in promoting AML progression, angiogenesis, and metastasis. The immune checkpoints (ICs) and metabolic processes are the key players in this process. In this review, we delineate the metabolic and immune checkpoint characteristics of the AML BMM, with a focus on the roles of BMM cells e.g. tumor-associated macrophages, natural killer cells, dendritic cells, metabolic profiles and related signaling pathways. We also discuss the signaling pathways stimulated in AML cells by BMM factors that lead to AML progression. We then delve into the roles of immune checkpoints in AML angiogenesis, metastasis, and cell proliferation, including co-stimulatory and inhibitory ICs. Lastly, we discuss the potential therapeutic approaches and future directions for AML treatment, emphasizing the potential of targeting metabolic and immune checkpoints in AML BMM as prognostic and therapeutic targets. In conclusion, the modulation of these processes through the use of directed drugs opens up new promising avenues in combating AML. Thereby, a comprehensive elucidation of the significance of these AML BMM cells' metabolic and immune checkpoints and signaling pathways on leukemic cells can be undertaken in the future investigations. Additionally, these checkpoints and cells should be considered plausible multi-targeted therapies for AML in combination with other conventional treatments in AML. Video Abstract.
Keywords: Acute myeloid leukemia; Angiogenesis; Bone marrow microenvironment; Cancer metabolism; Chemoresistance; Immune checkpoint; Metabolic checkpoint.
© 2023. BioMed Central Ltd., part of Springer Nature.
Conflict of interest statement
The authors declare no competing interests.
Figures
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

