Reducing gut microbiome-driven adipose tissue inflammation alleviates metabolic syndrome
- PMID: 37735685
- PMCID: PMC10512512
- DOI: 10.1186/s40168-023-01637-4
Reducing gut microbiome-driven adipose tissue inflammation alleviates metabolic syndrome
Abstract
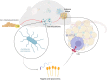
Background: The gut microbiota contributes to macrophage-mediated inflammation in adipose tissue with consumption of an obesogenic diet, thus driving the development of metabolic syndrome. There is a need to identify and develop interventions that abrogate this condition. The hops-derived prenylated flavonoid xanthohumol (XN) and its semi-synthetic derivative tetrahydroxanthohumol (TXN) attenuate high-fat diet-induced obesity, hepatosteatosis, and metabolic syndrome in C57Bl/6J mice. This coincides with a decrease in pro-inflammatory gene expression in the gut and adipose tissue, together with alterations in the gut microbiota and bile acid composition.
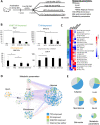
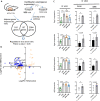
Results: In this study, we integrated and interrogated multi-omics data from different organs with fecal 16S rRNA sequences and systemic metabolic phenotypic data using a Transkingdom Network Analysis. By incorporating cell type information from single-cell RNA-seq data, we discovered TXN attenuates macrophage inflammatory processes in adipose tissue. TXN treatment also reduced levels of inflammation-inducing microbes, such as Oscillibacter valericigenes, that lead to adverse metabolic phenotypes. Furthermore, in vitro validation in macrophage cell lines and in vivo mouse supplementation showed addition of O. valericigenes supernatant induced the expression of metabolic macrophage signature genes that are downregulated by TXN in vivo.
Conclusions: Our findings establish an important mechanism by which TXN mitigates adverse phenotypic outcomes of diet-induced obesity and metabolic syndrome. TXN primarily reduces the abundance of pro-inflammatory gut microbes that can otherwise promote macrophage-associated inflammation in white adipose tissue. Video Abstract.
© 2023. BioMed Central Ltd., part of Springer Nature.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

