Protocol for a single-blind randomized clinical trial to test the efficacy of bilateral transcranial magnetic stimulation on upper extremity motor function in patients recovering from stroke
- PMID: 37735708
- PMCID: PMC10515042
- DOI: 10.1186/s13063-023-07584-7
Protocol for a single-blind randomized clinical trial to test the efficacy of bilateral transcranial magnetic stimulation on upper extremity motor function in patients recovering from stroke
Abstract
Background: No consensus currently exists regarding the optimal protocol for repetitive transcranial magnetic stimulation (rTMS) treatment of upper-extremity motor dysfunction after stroke. Studies have shown that combined low- and high-frequency stimulation (LF-HF-rTMS) of the bilateral cerebral hemispheres is more effective than sham stimulation or stimulation of one cerebral hemisphere alone in treating motor dysfunction in the subacute stage of stroke. The efficacy of this protocol in the convalescence phase of stroke has rarely been reported, and its mechanism of action has not been clarified. In this study, we designed a prospective, single-blind, randomized controlled trial to investigate the efficacy and safety of different stimulation regimens for the treatment of upper extremity motor disorders in patients with convalescent stage stroke and aimed to explore the underlying mechanisms based on biomarkers such as brain-derived neurotrophic factor (BDNF).
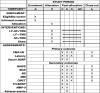
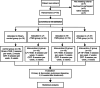
Methods: Seventy-six subjects will be randomly divided into combined, low-frequency, high-frequency, and control groups based on the proportion of 1:1:1:1, with 19 cases in each group. All groups will have conventional rehabilitation, on top of which the combined group will receive 1 Hz rTMS in the unaffected hemisphere and 10 Hz rTMS in the affected hemisphere. The low-frequency group will be administered 1 Hz rTMS in the unaffected hemisphere and sham stimulation in the contralateral hemisphere. The high-frequency group will be administered 10 Hz rTMS in the affected hemisphere and contralateral sham stimulation. The control group will receive bilateral sham stimulation. Assessments will be performed at baseline, after 2 weeks of treatment, and at post-treatment follow-up at week 6. The primary outcomes are FMA-UE (Fugl-Meyer assessment-upper extremity), latency, and serum BDNF levels. The secondary outcomes are the National Institute of Health Stroke Scale (NIHSS), Brunnstrom staging (BS), modified Ashworth scale (MAS), Modified Barthel Index (MBI), central motor conduction time (CMCT), precursor proteins of mature BDNF (proBDNF), and matrix metalloproteinase-9 (MMP-9) levels. Adverse events, such as headaches and seizures, will be recorded throughout the study.
Discussion: The findings of this study will help develop optimal stimulation protocols for motor recovery in stroke patients and identify biomarkers that respond to post-stroke motor rehabilitation, for better guidance of clinical treatment.
Trial registration: The study protocol was passed by the Medical Research Ethics Committee of the General Hospital of Ningxia Medical University on January 1, 2022 (no. KYLL-2021-1082). It was registered into the Chinese Clinical Trials Registry on May 22, 2022 (no. ChiCTR2200060201). This study is currently in progress.
Keywords: Combined; Cortical excitability; Plasticity; Serum factor; Stroke; rTMS.
© 2023. BioMed Central Ltd., part of Springer Nature.
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Effects of VR task-oriented training combined with rTMS on balance function and brain plasticity in stroke patients: a randomized controlled trial study protocol.Trials. 2024 Oct 21;25(1):702. doi: 10.1186/s13063-024-08519-6. Trials. 2024. PMID: 39434192 Free PMC article.
-
Effects of high-frequency repetitive transcranial magnetic stimulation over the contralesional motor cortex on motor recovery in severe hemiplegic stroke: A randomized clinical trial.Brain Stimul. 2020 Jul-Aug;13(4):979-986. doi: 10.1016/j.brs.2020.03.020. Epub 2020 Apr 2. Brain Stimul. 2020. PMID: 32380449 Clinical Trial.
-
Effects of corticospinal tract integrity on upper limb motor function recovery in stroke patients treated with repetitive transcranial magnetic stimulation.J Integr Neurosci. 2022 Mar 21;21(2):50. doi: 10.31083/j.jin2102050. J Integr Neurosci. 2022. PMID: 35364638 Clinical Trial.
-
Comparative Efficacy of Different Repetitive Transcranial Magnetic Stimulation Protocols for Stroke: A Network Meta-Analysis.Front Neurol. 2022 Jun 15;13:918786. doi: 10.3389/fneur.2022.918786. eCollection 2022. Front Neurol. 2022. PMID: 35785350 Free PMC article.
-
The effect and optimal parameters of repetitive transcranial magnetic stimulation on lower extremity motor function in stroke patient: a systematic review and meta-analysis.Disabil Rehabil. 2024 Oct;46(21):4889-4900. doi: 10.1080/09638288.2023.2283605. Epub 2023 Nov 22. Disabil Rehabil. 2024. PMID: 37991330
Cited by
-
Systematic review of repetitive transcranial magnetic stimulation for post-stroke hemiplegic shoulder pain.Neurol Sci. 2025 May;46(5):2007-2017. doi: 10.1007/s10072-024-07961-3. Epub 2025 Jan 2. Neurol Sci. 2025. PMID: 39745590 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

