Differential impact on motor unit characteristics across severities of adult spinal muscular atrophy
- PMID: 37735861
- PMCID: PMC10723249
- DOI: 10.1002/acn3.51906
Differential impact on motor unit characteristics across severities of adult spinal muscular atrophy
Abstract
Objective: To test the hypotheses that decomposition electromyography (dEMG) motor unit action potential (MUAP) amplitude and firing rate are altered in SMA; dEMG parameters are associated with strength and function; dEMG parameters are correlated with traditional electrophysiological assessments.
Methods: Ambulatory and non-ambulatory adults with SMA on nusinersen and healthy controls were enrolled. MUAPs were decomposed from multielectrode surface recordings during 30-s maximum contraction of the abductor digiti minimi (ADM). Isometric strength, upper limb function, patient-reported function, and standard electrophysiologic measures of the ADM (compound muscle action potential [CMAP], single motor unit potential [SMUP], motor unit number estimation [MUNE]) were collected.
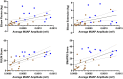
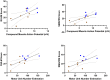
Results: dEMG MUAP amplitudes were higher in ambulatory versus control and non-ambulatory groups and were higher in controls versus non-ambulatory SMA. In contrast, dEMG firing rates were higher in ambulatory versus non-ambulatory and control groups but similar between non-ambulatory and control. dEMG parameters showed moderate to strong positive correlation with strength and function whereas CMAP and MUNE better correlated with function than strength. SMUP did not correlate with strength, function, or dEMG MUAP amplitude. dEMG parameters show overall good test-retest reliability.
Interpretation: dEMG provided reliable, noninvasive measure of MUAP amplitude size and firing rate and revealed divergent patterns across disease severity in adults with SMA. Firing rate enhancement, as seen in milder SMA, may provide a therapeutic avenue for improving function in more severe SMA, where firing rates appear preserved. MUAP amplitude size and firing rate, quantified with dEMG, may be promising monitoring biomarker candidates for noninvasive assessment of SMA.
© 2023 The Authors. Annals of Clinical and Translational Neurology published by Wiley Periodicals LLC on behalf of American Neurological Association.
Conflict of interest statement
KMK: Advisory board for Biogen, member of Neuromuscular Study Group Planning Committee, travel support from Neuromuscular Study Group; SH: salary support from Muscular Dystrophy Association; SJK: consulting for Novartis; BE: research funding from Biogen, Genentech, Alexion, Pharnext, Viela Bio, advisory board for Biogen, Genentech, Argenx, Honoraria from Muscular Dystrophy Association, Stanford University, Travel Support from Cure SMA, Muscular Dystrophy Association, Committee Member for Cure SMA, receipt of drug for clinical trial from Celgene; WDA: research funding from NMD Pharma, Avidity Biosciences, Consulting for NMD Pharma, Avidity Biosciences, Dyne Therapeutics, Novartis, La Hoffman Roche, Genentech, Design Therapeutics, Cadent Therapeutics, Catalyst Pharmaceuticals, Honoraria from University of Rochester, Travel Support from NMD Pharma, Chair/Member of Novartis Data Safety and Monitoring Board, Chair of Neuromuscular Study Group Planning Committee, Patent for “Methods and compositions for treating disorders and diseases using Survival Motor Neuron (SMN) protein.”
Figures







References
-
- Lefebvre S, Burglen L, Reboullet S, et al. Identification and characterization of a spinal muscular atrophy‐determining gene. Cell. 1995;80(1):155‐165. - PubMed
-
- Lefebvre S, Burlet P, Liu Q, et al. Correlation between severity and SMN protein level in spinal muscular atrophy. Nat Genet. 1997;16(3):265‐269. - PubMed
-
- Mendell JR, Al‐Zaidy S, Shell R, et al. Single‐dose gene‐replacement therapy for spinal muscular atrophy. N Engl J Med. 2017;377(18):1713‐1722. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
