Human macrophage migration inhibitory factor potentiates mesenchymal stromal cell efficacy in a clinically relevant model of allergic asthma
- PMID: 37735872
- PMCID: PMC10638061
- DOI: 10.1016/j.ymthe.2023.09.013
Human macrophage migration inhibitory factor potentiates mesenchymal stromal cell efficacy in a clinically relevant model of allergic asthma
Abstract
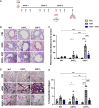
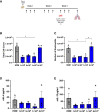
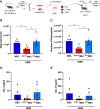
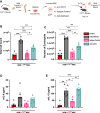
Current asthma therapies focus on reducing symptoms but fail to restore existing structural damage. Mesenchymal stromal cell (MSC) administration can ameliorate airway inflammation and reverse airway remodeling. However, differences in patient disease microenvironments seem to influence MSC therapeutic effects. A polymorphic CATT tetranucleotide repeat at position 794 of the human macrophage migration inhibitory factor (hMIF) gene has been associated with increased susceptibility to and severity of asthma. We investigated the efficacy of human MSCs in high- vs. low-hMIF environments and the impact of MIF pre-licensing of MSCs using humanized MIF mice in a clinically relevant house dust mite (HDM) model of allergic asthma. MSCs significantly attenuated airway inflammation and airway remodeling in high-MIF-expressing CATT7 mice but not in CATT5 or wild-type littermates. Differences in efficacy were correlated with increased MSC retention in the lungs of CATT7 mice. MIF licensing potentiated MSC anti-inflammatory effects at a previously ineffective dose. Mechanistically, MIF binding to CD74 expressed on MSCs leads to upregulation of cyclooxygenase 2 (COX-2) expression. Blockade of CD74 or COX-2 function in MSCs prior to administration attenuated the efficacy of MIF-licensed MSCs in vivo. These findings suggest that MSC administration may be more efficacious in severe asthma patients with high MIF genotypes (CATT6/7/8).
Keywords: allergic asthma; cyclooxygenase; house dust mite; macrophage migration inhibitory factor; mesenchymal stromal cells.
Copyright © 2023 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures





Comment in
-
Macrophages, MIFs, and MSCs: Defining an MOA in murine experimental asthma.Mol Ther. 2023 Nov 1;31(11):3117-3118. doi: 10.1016/j.ymthe.2023.10.010. Epub 2023 Oct 20. Mol Ther. 2023. PMID: 37865098 Free PMC article.
Similar articles
-
The VEGF-Mediated Cytoprotective Ability of MIF-Licensed Mesenchymal Stromal Cells in House Dust Mite-Induced Epithelial Damage.Eur J Immunol. 2025 Jan;55(1):e202451205. doi: 10.1002/eji.202451205. Epub 2024 Nov 6. Eur J Immunol. 2025. PMID: 39502000 Free PMC article.
-
Blockade of MIF biological activity ameliorates house dust mite-induced allergic airway inflammation in humanized MIF mice.FASEB J. 2023 Aug;37(8):e23072. doi: 10.1096/fj.202300787R. FASEB J. 2023. PMID: 37498233
-
Mesenchymal stromal cells dampen trained immunity in house dust mite-primed macrophages expressing human macrophage migration inhibitory factor polymorphism.Cytotherapy. 2024 Oct;26(10):1245-1251. doi: 10.1016/j.jcyt.2024.05.010. Epub 2024 May 19. Cytotherapy. 2024. PMID: 38819366
-
The Role of MIF on Eosinophil Biology and Eosinophilic Inflammation.Clin Rev Allergy Immunol. 2020 Feb;58(1):15-24. doi: 10.1007/s12016-019-08726-z. Clin Rev Allergy Immunol. 2020. PMID: 30680604 Review.
-
Macrophage migration inhibitory factor: gene polymorphisms and susceptibility to inflammatory diseases.Clin Infect Dis. 2005 Nov 15;41 Suppl 7:S513-9. doi: 10.1086/432009. Clin Infect Dis. 2005. PMID: 16237655 Review.
Cited by
-
Proinflammatory cytokines sensitise mesenchymal stromal cells to apoptosis.Cell Death Discov. 2025 Mar 28;11(1):121. doi: 10.1038/s41420-025-02412-0. Cell Death Discov. 2025. PMID: 40148285 Free PMC article.
-
Damage of the Bone Marrow Stromal Precursors in Patients with Acute Leukemia at the Onset of the Disease and During Treatment.Int J Mol Sci. 2024 Dec 11;25(24):13285. doi: 10.3390/ijms252413285. Int J Mol Sci. 2024. PMID: 39769050 Free PMC article.
-
The MSC-EV-microRNAome: A Perspective on Therapeutic Mechanisms of Action in Sepsis and ARDS.Cells. 2024 Jan 9;13(2):122. doi: 10.3390/cells13020122. Cells. 2024. PMID: 38247814 Free PMC article. Review.
-
The VEGF-Mediated Cytoprotective Ability of MIF-Licensed Mesenchymal Stromal Cells in House Dust Mite-Induced Epithelial Damage.Eur J Immunol. 2025 Jan;55(1):e202451205. doi: 10.1002/eji.202451205. Epub 2024 Nov 6. Eur J Immunol. 2025. PMID: 39502000 Free PMC article.
-
Carbon monoxide licensing of MSCs enhances their efficacy through autophagy-mediated miRNA mechanisms.Mol Ther. 2024 Jul 3;32(7):2047-2049. doi: 10.1016/j.ymthe.2024.06.008. Epub 2024 Jun 20. Mol Ther. 2024. PMID: 38906151 Free PMC article. No abstract available.
References
-
- Sverrild A., Hansen S., Hvidtfeldt M., Clausson C.M., Cozzolino O., Cerps S., Uller L., Backer V., Erjefält J., Porsbjerg C. The effect of tezepelumab on airway hyperresponsiveness to mannitol in asthma (UPSTREAM) Eur. Respir. J. 2022;59 - PubMed
-
- Bleecker E.R., FitzGerald J.M., Chanez P., Papi A., Weinstein S.F., Barker P., Sproule S., Gilmartin G., Aurivillius M., Werkström V., et al. Efficacy and safety of benralizumab for patients with severe asthma uncontrolled with high-dosage inhaled corticosteroids and long-acting β2-agonists (SIROCCO): a randomised, multicentre, placebo-controlled phase 3 trial. Lancet Lond. Engl. 2016;388:2115–2127. - PubMed
-
- Corren J., Castro M., O’Riordan T., Hanania N.A., Pavord I.D., Quirce S., Chipps B.E., Wenzel S.E., Thangavelu K., Rice M.S., et al. Dupilumab efficacy in patients with uncontrolled, moderate-to-severe allergic asthma. J. Allergy Clin. Immunol. Pract. 2020;8:516–526. - PubMed
-
- Ortega H.G., Yancey S.W., Mayer B., Gunsoy N.B., Keene O.N., Bleecker E.R., Brightling C.E., Pavord I.D. Severe eosinophilic asthma treated with mepolizumab stratified by baseline eosinophil thresholds: a secondary analysis of the DREAM and MENSA studies. Lancet Respir. Med. 2016;4:549–556. - PubMed
-
- Chan R., Lipworth B. Efficacy of biologic therapy on airway hyperresponsiveness in asthma. Ann. Allergy Asthma Immunol. Off Publ. Am. Coll. Allergy Asthma Immunol. 2023:00121–00127. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

