CXCR4-modified CAR-T cells suppresses MDSCs recruitment via STAT3/NF-κB/SDF-1α axis to enhance efficacy against pancreatic cancer
- PMID: 37735875
- PMCID: PMC10638076
- DOI: 10.1016/j.ymthe.2023.09.010
CXCR4-modified CAR-T cells suppresses MDSCs recruitment via STAT3/NF-κB/SDF-1α axis to enhance efficacy against pancreatic cancer
Abstract
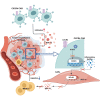
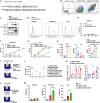
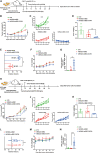
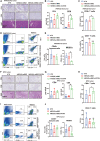
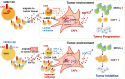
Claudin18.2 (CLDN18.2)-specific chimeric antigen receptor (CAR-T) cells displayed limited efficacy in CLDN18.2-positive pancreatic ductal adenocarcinoma (PDAC). Strategies are needed to improve the trafficking capacity of CLDN18.2-specific CAR-T cells. PDAC has a unique microenvironment that consists of abundant cancer-associated fibroblasts (CAFs), which could secrete stromal cell-derived factor 1α (SDF-1α), the ligand of CXCR4. Then, we constructed and explored CLDN18.2-targeted CAR-T cells with CXCR4 co-expression in treating immunocompetent mouse models of PDAC. The results indicated that CXCR4 could promote the infiltration of CAR-T cells and enhance their efficacy in vivo. Mechanistically, the activation of signal transducer and activator of transcription 3 (STAT3) signaling was impaired in CXCR4 CAR-T cells, which reduced the release of inflammatory factors, such as tumor necrosis factor-α, IL-6, and IL-17A. Then, the lower release of inflammatory factors suppressed SDF-1α secretion in CAFs via the nuclear factor κB (NF-κB) pathway. Therefore, the decreased secretion of SDF-1α in feedback decreased the migration of myeloid-derived suppressor cells (MDSCs) in tumor sites. Overall, our study demonstrated that CXCR4 CAR-T cells could traffic more into tumor sites and also suppress MDSC migration via the STAT3/NF-κB/SDF-1α axis to obtain better efficacy in treating CLDN18.2-positive pancreatic cancer. Our findings provide a theoretical rationale for CXCR4 CAR-T cell therapy in PDAC.
Keywords: CAFs; CAR-T cells; MDSCs; PDAC; SDF-1α; cancer-associated fibroblasts; chimeric antigen receptor; myeloid-derived suppressor cells; pancreatic ductal adenocarcinoma; stromal cell-derived factor 1α.
Copyright © 2023 The American Society of Gene and Cell Therapy. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests H.J. and Z.L. have ownership interests of CAR-T cells relating to this work and are stockholders in CARsgen Therapeutics.
Figures



References
-
- Porter D.L., Hwang W.T., Frey N.V., Lacey S.F., Shaw P.A., Loren A.W., Bagg A., Marcucci K.T., Shen A., Gonzalez V., et al. Chimeric antigen receptor T cells persist and induce sustained remissions in relapsed refractory chronic lymphocytic leukemia. Sci. Transl. Med. 2015;7:303ra139. - PMC - PubMed
-
- Shi D., Shi Y., Kaseb A.O., Qi X., Zhang Y., Chi J., Lu Q., Gao H., Jiang H., Wang H., et al. Chimeric Antigen Receptor-Glypican-3 T-Cell Therapy for Advanced Hepatocellular Carcinoma: Results of Phase I Trials.(2020) Clin. Cancer Res. 2020;26:3979–3989. - PubMed
-
- Neelapu S.S., Locke F.L., Go W.Y. CAR T-Cell Therapy in Large B-Cell Lymphoma. N. Engl. J. Med. 2018;378:1065. - PubMed
-
- Sun Y. Tumor microenvironment and cancer therapy resistance. Cancer Lett. 2016;380:205–215. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

