Case Report: Unedited allogeneic chimeric antigen receptor T cell bridging to conditioning-free hematopoietic stem cell transplantation for a child with refractory Burkitt lymphoma
- PMID: 37736096
- PMCID: PMC10510403
- DOI: 10.3389/fimmu.2023.1219872
Case Report: Unedited allogeneic chimeric antigen receptor T cell bridging to conditioning-free hematopoietic stem cell transplantation for a child with refractory Burkitt lymphoma
Abstract
Purpose: Burkitt lymphoma (BL) is the most common tumor of non-Hodgkin's lymphoma (NHL) in children, accounting for about 40% of cases. Although different combined short-course chemotherapies have achieved a good effect, refractory/relapsed BL has a poor prognosis with cure rates less than 30%. Chimeric antigen receptor T cell (CAR-T) therapy has developed rapidly in recent years and achieved excellent results in acute lymphoblastic leukemia (ALL). However, in some cases, there is a failure to produce autologous CAR-T cells because of T-cell dysfunction. In such cases, allogeneic CAR-T therapy has to be considered.
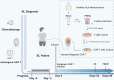
Methods: A 17-year-old boy with stage II BL did not respond to extensive chemotherapy and sequential autologous CAR-T therapy. Lentiviral vectors containing anti-CD20-BB-ζ (20CAR) and anti-CD22-BB-ζ (22CAR) transgenes were used to modify the T cells from an HLA-identical matched unrelated donor. Flow cytometry was used to assess the cytokine analyses and CAR-T cell persistence in peripheral blood, enumerated by qPCR as copies per ug DNA. Informed consent for autologous/allogeneic CAR-T therapy was obtained from the patient and his legal guardian.
Results: Unedited HLA-matched allogeneic CD20 and CD22 CAR-T cells were infused after lymphodepletion chemotherapy with cyclophosphamide and fludarabine. The patient experienced Grade IV cytokine release syndrome (CRS) and went into complete remission (CR) after anti-inflammatory treatment including tocilizumab. Because of persistent pancytopenia and full donor chimerism, the same donor's conditioning-free peripheral blood stem cells were successfully transplanted 55 days post CAR-T. Neutrophils were engrafted at day +11 and platelets were rebuilt at day +47 without obvious acute graft-versus-host disease (GVHD), but there was mild chronic GVHD in the skin and eyes. Currently, active anti-rejection therapy is still underway.
Conclusion: Unedited HLA-matched allogeneic CAR-T cell therapy could be an innovative, effective, and safe treatment for children with refractory/relapse BL without obvious acute GVHD. Conditioning-free allogeneic hematopoietic stem cell transplantation (HSCT) from the same donor is feasible for a patient with full donor T-cell chimerism after allogeneic CAR-T. It cannot be ignored that close GVHD monitoring is needed post HSCT.
Keywords: Burkitt lymphoma; CAR T-Cell Therapy; allogeneic cells; graft-versus-host disease; hematopoietic stem cell transplantation.
Copyright © 2023 Yang, Luo, Qian, Huang, Zhang, Wang, Luo, Qin, Li and Chen.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Lee DW, Kochenderfer JN, Stetler-Stevenson M, Cui YK, Delbrook C, Feldman SA, et al. . T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet (2015) 385(9967):517–28. doi: 10.1016/S0140-6736(14)61403-3 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

